Description
What is Equipment Qualification?
The design, construction and delivery of manufacturing systems and equipment into a facility requires a systematic approach in order to meet the user requirements and that of the relevant authorities like cGMP, local governing codes, law and regulations.
Equipment Qualification is the action of proving and documenting that equipment and ancillary systems are properly installed, work correctly, and actually lead to the expected results. This is to ensure that all critical aspects or acceptance criteria for its intended use are met. Qualification is part of the validation process, a regulatory compliance requirement.
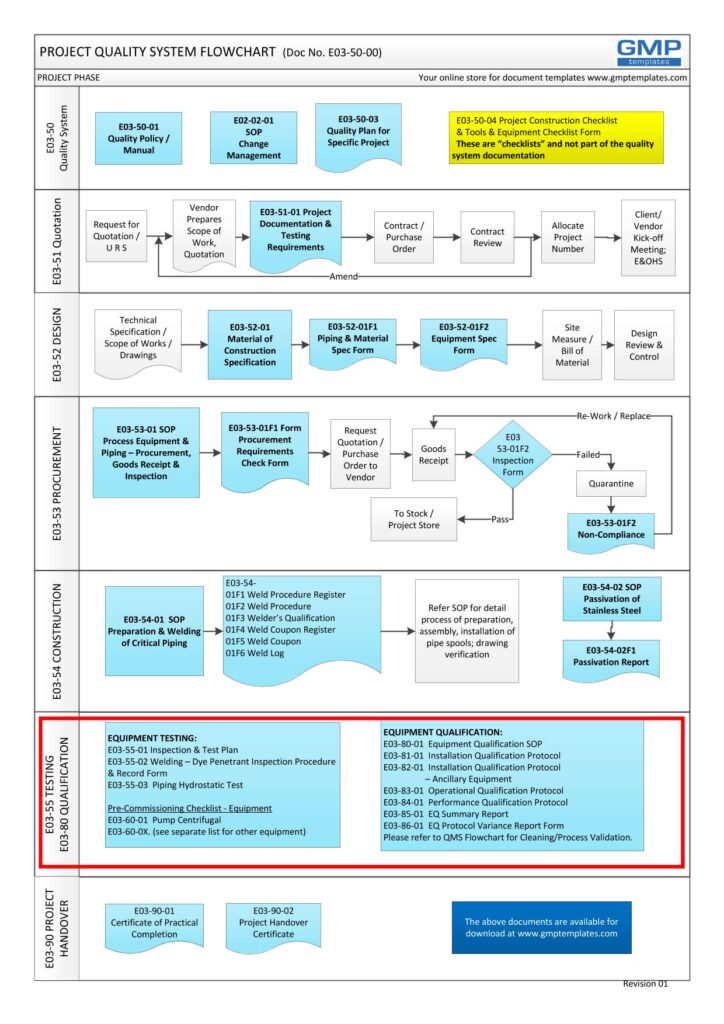
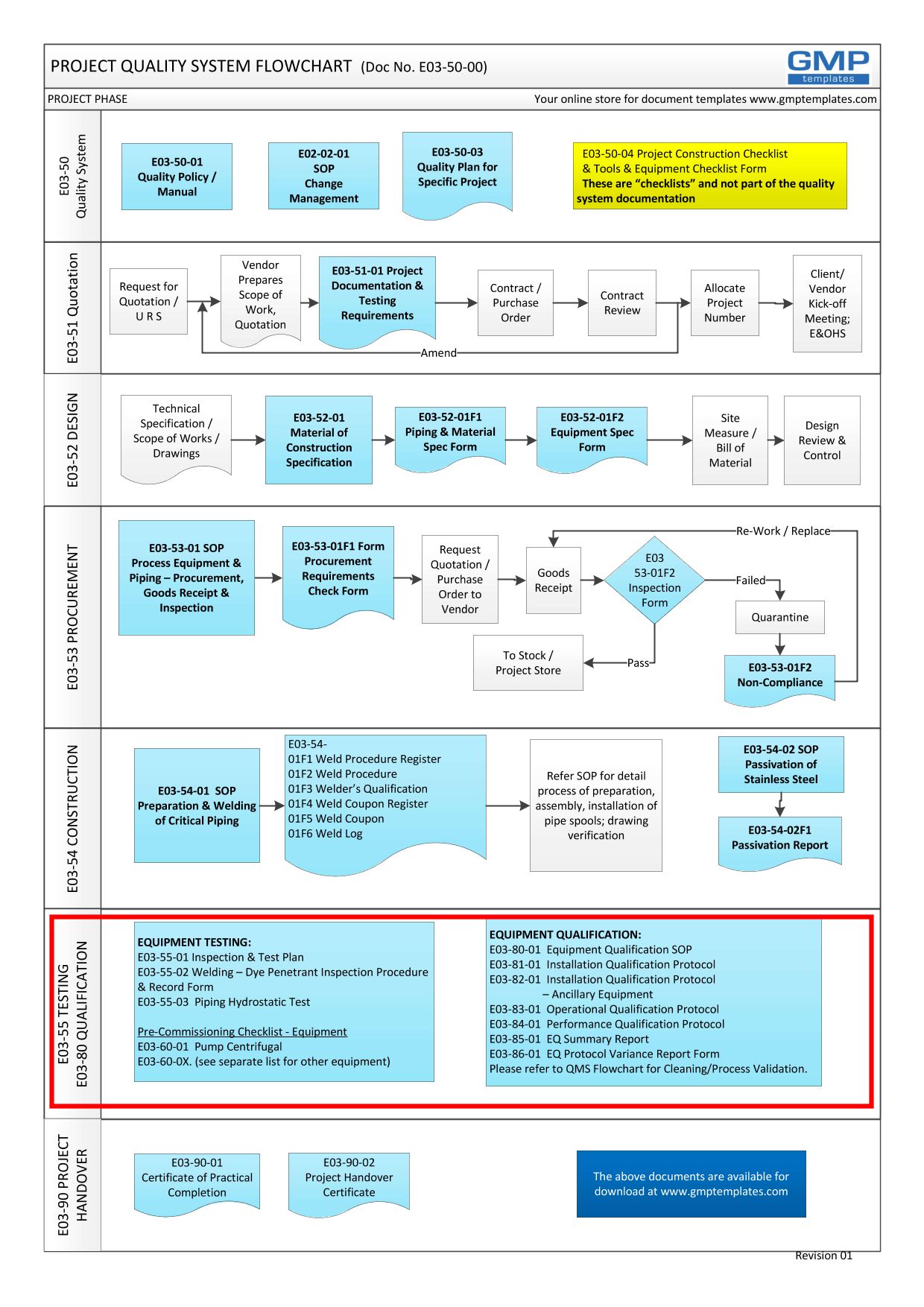
E03-50-00 Project Quality System Flowchart – Testing, commissioning and Qualification (Validation)
What is this document about?
The purpose of this SOP is to outline the procedures for performing Equipment Qualification of equipment and piping.
The three components of the Equipment Qualification are:
- Installation Qualification (IQ) – documented verification that the equipment or systems, as installed or modified, comply with the approved design, the manufacturer’s recommendations and/or user requirements. Source: PIC/S
- Operational Qualification (OQ) – documented verification that the equipment or systems, as installed or modified, perform as intended throughout the anticipated operating ranges. Source: PIC/S
- Performance Qualification (PQ) – documented verification that the equipment and ancillary systems, as connected together, can perform effectively and reproducibly based on the approved process method and specifications. Source: PIC/S
This document is the Standard Operating Procedure for Equipment Qualification and does not include the IQ, OQ or PQ protocol templates
This procedure plays a role in two areas:
- GEP – is part of the Testing process in a Project Quality System (see flowchart below)
- QMS – is a component of Facility & Equipment under Resource Requirements (refer QMS Flowchart)
This well proven document has been used in numerous GMP facilities
The document has two Parts (a) introduction to template – 4 pages (b) a 8-page template + attachments
Attachments & Forms included:
- E03-80-01F1 Qualification Summary Report (4 pages) – guides you through what is to be included in the report
- E03-80-01F2 Protocol Variance Report Form (2 pages) – it guides you through a typical deviation investigation and CAPA process to close the variance
It includes a process map of the sequence of events from design, impact assessment to qualification
Both prospective and retrospective qualifications are included
To view and PRINT the above flowchart in PDF format – E03-50-00_Project_Quality_Sys_Flowchart
Associated Industry/regulatory guidance and requirements:
- ISPE Baseline Guide – Commissioning and Qualification
- FDA CFR Part 211 Subpart C (Building and Facilities) and Subpart D (Equipment)
- PIC/S Guide to GMP for Medicinal Products Part I (Clause 1.4/xi) and II (12.3 Qualification)
- ASTM E2500 Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing System and Equipment
Included Sections:
Purpose, Scope, References and Associated Documents, Definitions /Acronyms, Responsibility, Procedures, Attachments.
Keywords:
Equipment Qualification Installation Qualification (IQ) Operational Qualification (OQ) Performance Qualification (PQ)
Who needs this document?
- Vendors – equipment fabricators and manufacturers; mechanical piping contractors
- Any department who uses equipment & utilities in R&D, manufacturing, testing, laboratories.
- Projects, Validation, Engineering department
- Quality Control
- Manufacturing
Related Documents:
E03-50-00 Project Quality System Flowchart
E03-50-01 Project Quality System Policy
E03-50-02 Project Change Control
E03-50-03 Project & Quality Plan (Concise)
E03-50-04 Project Construction Checklist
E03-51-01 Project Documentation & Testing Requirements
E03-52-01 Material of Construction Specification
E03-53-01 Project Procurement & Inspection Procedure
E03-54-01 Preparation & Welding of Critical Piping
E03-54-02 Passivation of Stainless Steel & Record Form
EQUIPMENT TESTING & QUALIFICATION:
E03-55-01 Inspection & Test Plan (Form)
E03-55-02 Welding – Dye Penetrant Inspection Procedure & Record Form
E03-55-03 Piping Hydrostatic Test Procedure & Form
E03-60-XX Pre-Commissioning Checklist – various equipment
E03-60-01 Pre-Commissioning Checklist – Pump (Centrifugal)
E03-80-01 Equipment Qualification SOP – this document
E03-80-01F1 Qualification Summary Report
E03-80-01F2 Protocol Variance Report Form
E03-81-01 IQ Protocol Template
E03-82-01 IQ Protocol Template For Ancillary Equipment
E03-83-01 OQ Protocol Template
E03-84-01 PQ Protocol Template
E02-90-01 Project Handover – O&M Manual