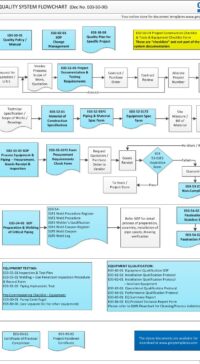
Description
Why do you need to control Changes in GMP Documentation?
- An effective Change Management System (or Change Control) is a must for smooth implementation of continuous improvements in a controlled manner to your GMP operations. This SOP is a subset of the Change Management System where GMP documents are affected by the change:
- changes to existing SOP’s or Policies or Raw Material Specifications or any GMP Documents
- new documentation may need to be created and implemented,
- or existing document/s need to be made obsolete
- All changes that may have an impact on product quality requires proper control to ensure each step of the change is effective, traceable and can be verified or validated.
- This SOP covers all the processes and relevant documents affected by the initial Change Control process through to close out of the Change.
- GMP Documentation – range from Regulatory submission to Product Release.
- All regulatory authorities require implementation of a change management system.
PURPOSE:
The purpose of this procedure is to outline the process for the preparation, review, approval, issuance, distribution, revision, retrieval, destruction and obsolescence of GMP documents.
There are 2 parts to this package:
- Part One – introduction and reference to Regulatory clauses and requirements
- Part Two – comprehensive 16-page SOP + 4 forms (the Table of Contents is shown below)
Click on the Table of Contents image to view a larger image
Who needs this procedure?
All personnel involved with GMP change in process or system.
Related Documents/Process:
Q00-01 Policy Statement – Quality Management System including flowchart
Q04-0110 Change Control SOP
E02-0201 Engineering Change Control