GEP is one of the foundations on which other processes and systems are based eg Equipment Qualification, Validation, GxP, GAMP, GMP and Quality Management System.
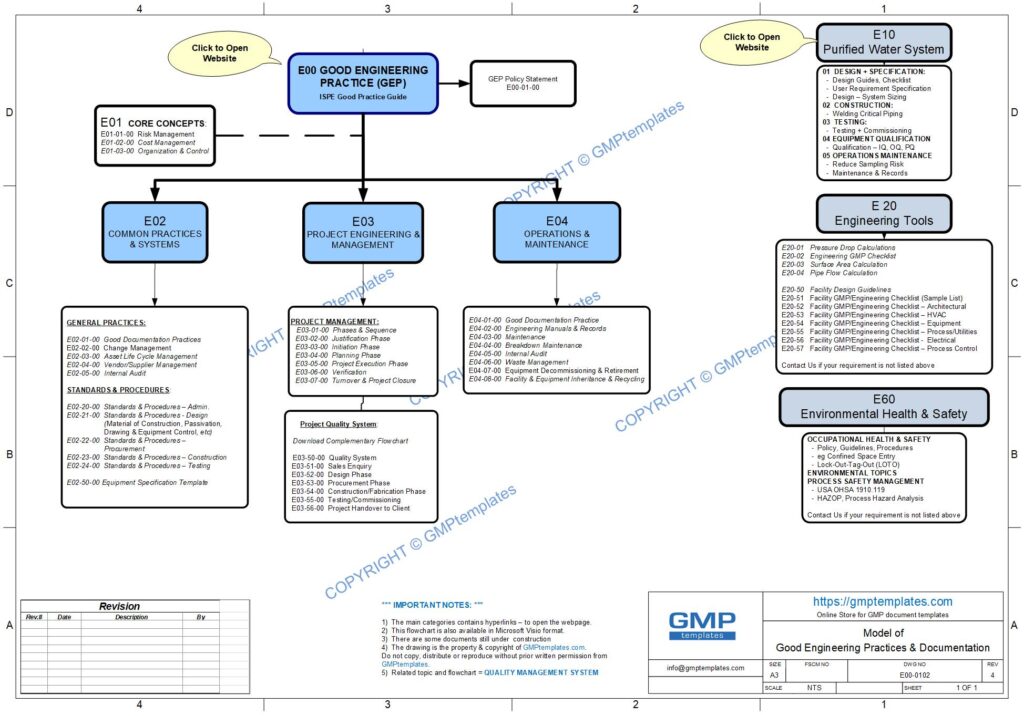
E00-0102 Good Engineering Practice (GEP) Flowchart (jpg)
If implemented well – GEP will provide improvement in use of resources, cost, schedule, quality, performance, safety, environment or other measurable factors that impact the health of an organization.
Download a complementary GOOD ENGINEERING PRACTICE model.
ISPE (International of Pharmaceutical Engineering www.ispe.org ) carried out an Engineering Standards Benchmarking (ESB) survey (2012) for members:
- 79.1% responded – Improved consistency among engineering deigns
- 77.9% responded – Improved quality of project deliverables
- other favourable responses are (a) Reduced Engineering Costs (b) Reduced Project Delivery Timeline, etc
As an ex-colleague always reminded the team “THE HARD BIT IS MAKING IT EASY”
What is your opinion to introduce GEP in your practices and how effective would you estimate it to be?



