Your online store for Good Manufacturing Practices (GMP) document templates:
- Quality Management System QMS
- Good Engineering Practices GEP & Other Topics
OUR PRODUCTS AND SERVICES
- “Good Manufacturing Practices (GMP, also referred to as ‘cGMP’ or ‘current Good Manufacturing Practice’) is the aspect of quality assurance that ensures that medicinal products are consistently produced and controlled to the quality standards appropriate to their intended use and as required by the product specification.” Source= WHO (World Health Organization)
- “… Adherence to the CGMP regulations assures the identity, strength, quality, and purity of drug products by requiring that manufacturers of medications adequately control manufacturing operations. This includes establishing strong quality management systems, …….” Source= FDA Facts about cGMP
- We are your resource centre for Good Manufacturing Practices (GMP) document templates for Life Science, FMCG & Critical Facilities:
- Policies, Standard Operating Procedures (SOP), Work Instructions, Checklists, Systems and Forms
- Engineering Best Practices, Tools and Calculations
- Guidelines and Whitepapers
- Topics include: (Our templates are grouped into Main Categories and Sub-Categories)
MAIN CATEGORIES:
-
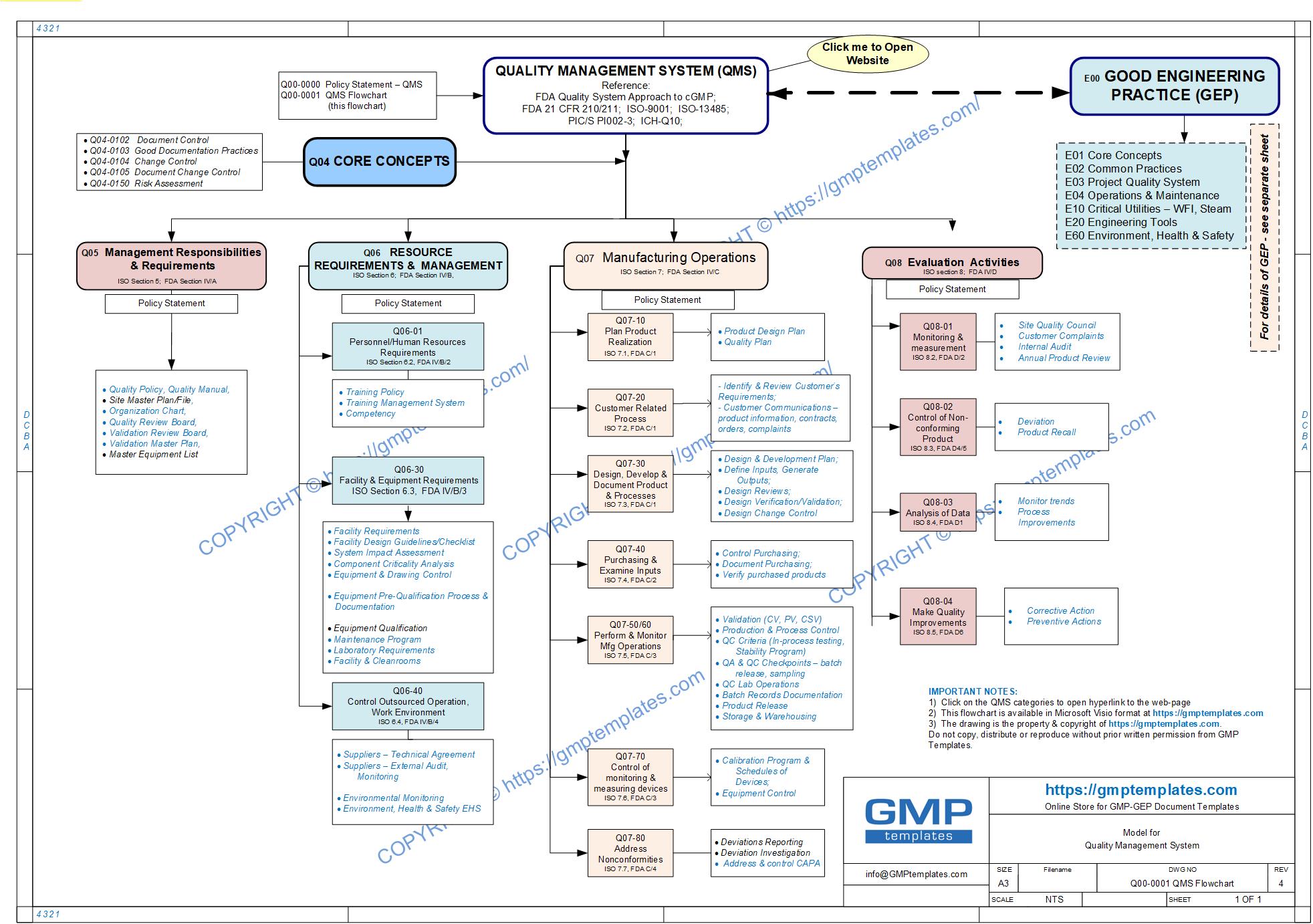
- Quality Management System (QMS) based on:
- FDA, PIC/S, ICH-Q10, EU, ISO-9001, ISPE, ASTM, WHO, TGA
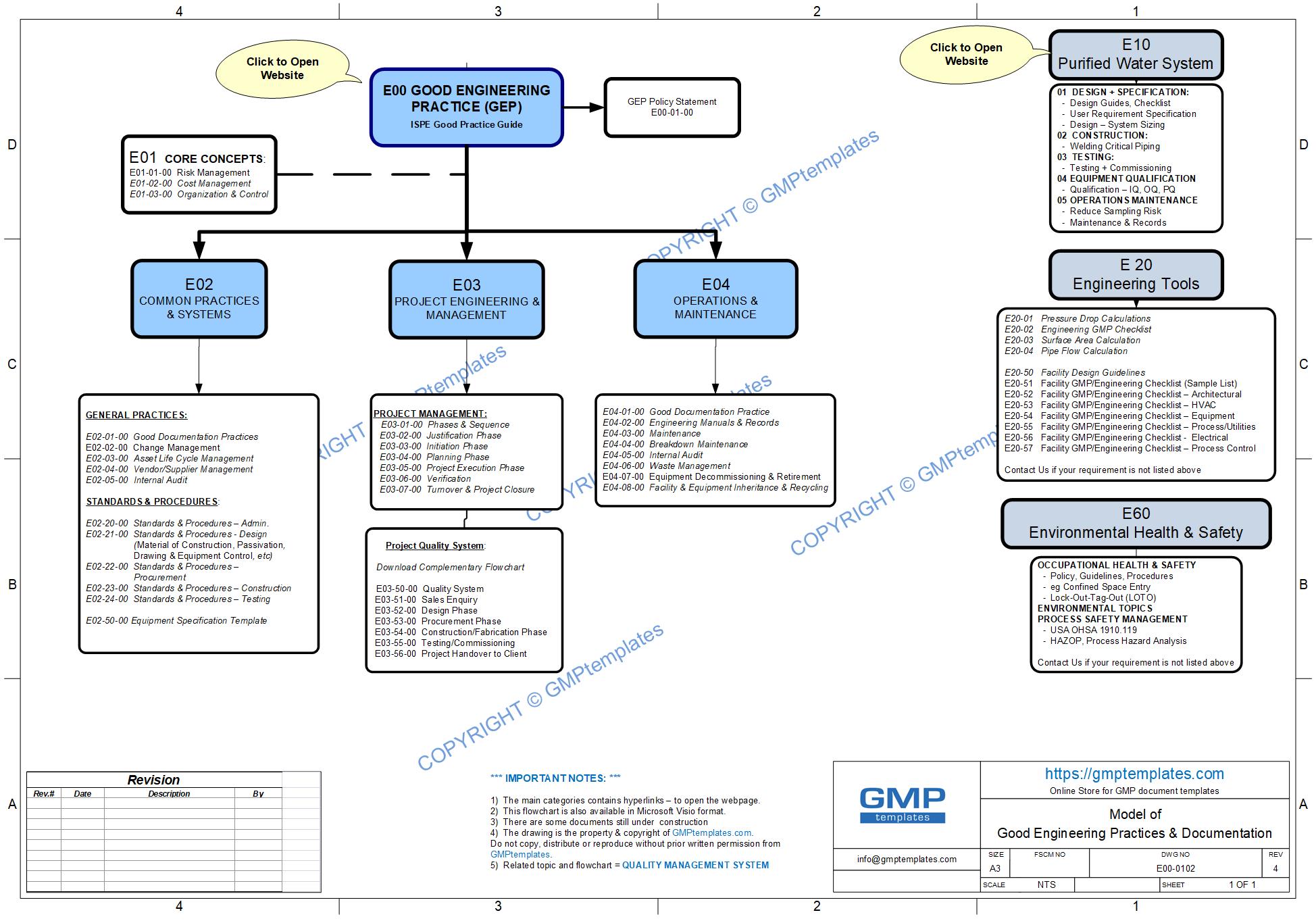
- Good Engineering Practice (GEP) inline with International Society of Pharmaceutical Engineering (ISPE)
- Purified Water System (WFI) – design, construction, testing, operation & maintenance – inline with US/European Pharmacopoeia (USP, EP)
- GMP Facility Design & Construction Guidelines
- Engineering Standards and Systems.
- Environmental Health & Safety (EHS)
- Quality Management System (QMS) based on:
Where Do I start?
- Is there a specific topic you are looking for – do a Search
- For an ENGINEERING topic – there are two options:
- open, download and print the FREE GEP (GOOD ENGINEERING PRACTICE) Flowchart. Use the flowchart as a reference for the next step. The PDF flowchart contains “Hyperlinks” to open the Webpage.
- Alternately – Go to “PRODUCTS – MAIN CATEGORIES” (below)
- For a QUALITY ASSURANCE (QA) or organizational topic –
- open, download and print the FREE QMS (Q00-0001 Quality Management System Flowchart) Flowchart. Use the flowchart (roadmap) as a reference for the next step. The PDF flowchart contains “Hyperlinks” to open the Webpage.
- Go to “PRODUCTS – MAIN CATEGORIES” (below)
- Contact Us – if you need some help or email [email protected]
Click on Flowcharts to open the PDF file.
The “CATEGORIES” in the PDF Flowcharts contain “hyperlinks” to open the corresponding Webpage.
The “CATEGORIES” in the PDF Flowcharts contain “hyperlinks” to open the corresponding Webpage.
PRODUCTS – MAIN CATEGORIES (by Topics)
Our GMP Templates:
- Industry proven document templates & forms
– have been implemented in companies from multinationals to small start-up companies. - Our document templates cater for both Client (manufacturers) and Equipment Vendors, Contractors, Suppliers, Service Providers.
- Using of templates and guidelines will reduce the amount of time the user needs to spend on researching and developing any process and document within a GMP environment.
- Reasons to buy our templates:
- to give you a quick starting point for your final document
- most of our documents will give you options to benchmark your standard against industry’s
- Most of our documents have been implemented in established regulated facilities – this can be used to set the basis of your document.
- Reasons to buy our templates: