E00 Good Engineering Practice (GEP)
WHAT IS GEP?
Good Engineering Practice (GEP) is established methods and standards that can be applied throughout an engineering activity, project or equipment life-cycle to ensure delivery of appropriate and cost effective solutions.
WHY DO WE NEED GEP?
GEP is one of the foundations on which other processes and systems are based eg Equipment Qualification, Validation, GxP, GAMP, GMP and Quality Management System.
If implemented well – GEP will provide improvement in use of resources, cost, schedule, quality, performance, safety, environment or other measurable factors that impact the health of an organization.
GEP is one of the key concepts to ensure the introduction of equipment and systems into facility meets user and regulatory requirements while being cost-effective, compliant and well documented.
Components of GEP are:
- E00 Policy Statement
- E01 Core Concepts to all the process, systems and standards that make up GEP are risks, cost and organizational control management
- E02 Common Practices and Systems are procedures and standards relevant to design, testing, quality control, operations and maintenance of engineering process and equipment
- E03 Project Engineering and Management – are standard processes and procedures relevant to projects (both GMP & non-GMP projects). There is a separate section in developing a Project Quality System
- E04 Operations and Maintenance are standards processes and procedures for equipment and facility.
We have shown these categories graphically in our GEP Flowchart (below):
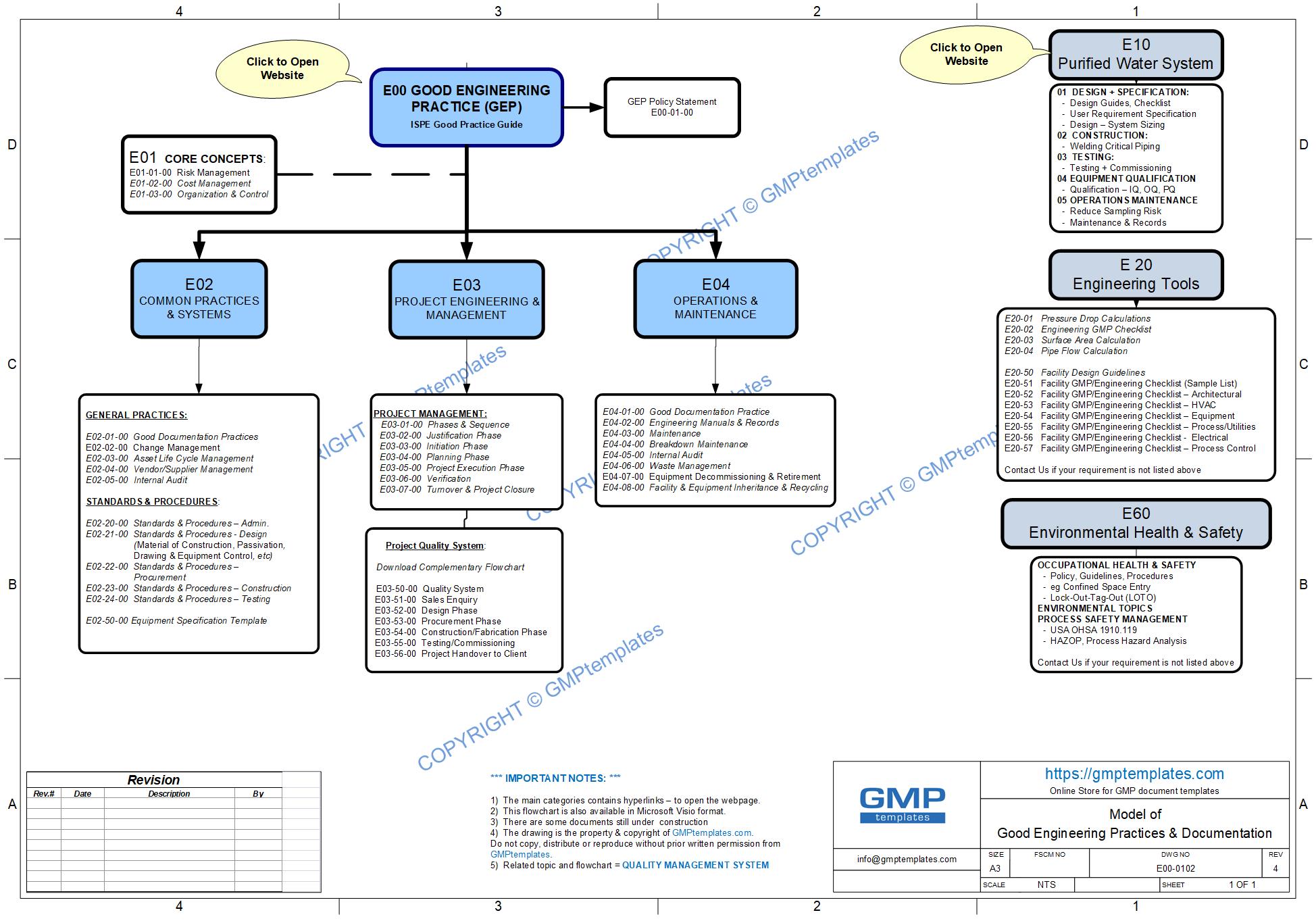
E00-0102 Good Engineering Practice (GEP) Flowchart (jpg)
To Open-Print-Download a PDF of the flowchart –> E00-0102 Good Engineering Practice Flowchart-r3
INDUSTRY TREND:
As pharmaceutical manufacturers confront increased margin pressure, new ways to lower their costs and instigate process improvements need to be implemented. Equipment Qualification and Validation costs are blown out of proportion. The trend is to start with the first principles of engineering and build a solid foundation for compliance and product quality.
In 2007, ASTM together with a consensus input from industry, ISPE, FDA, EMEA and GAMP developed and published E2500 – Standard Guide for Specification, Design and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems and Equipment.
ASTM E2500 emphasized that “6.6.1 Good Engineering Practice (GEP) should underpin and support the specification, design, and verification activities”.
In 2008, ISPE instigated and published a good practice guide “Good Engineering Practice”.
ISPE has been collaborating with the ASME Bioprocess Equipment Committee in the development of a standard definition for data and document requirements associated with new assets. The first effort “Vendor Documentation Requirements for New Instruments,” was published as an appendix in the latest edition of the ASME BPE Standard.
OUR WEBSITE OFFERS:
Our Team at www.gmptemplates.com, with combined decades of experience will ease your effort in the implementation of GEP by offering industry proven procedures and standards.
YOUR NEXT STEP TO ESTABLISH GEP:
State your Company’s philosophy and commitment to implement Good Engineering Practices (GEP). Then define the importance of Good Engineering Practices in the execution of engineering activities or projects to the introduction of manufacturing systems and equipment. Our E00-01-01 Policy Statement on GEP is the first document to kick-off the process. CLICK HERE to view more details.
View and understand our GEP Flowchart and translate to your organization’s requirements
Applying a “risk-based” approach, highlight and prioritize the procedures, methods and standards that you wish to introduce to your facility. Then select from our library of document templates.
If you need help, please use the Enquiry Form in Contact Us.
The SUB-CATEGORIES of Good Engineering Practice (GEP) are shown below.
CLICK TO OPEN SUB-CATEGORY: