Q00 QUALITY MANAGEMENT SYSTEM (QMS)
WHAT IS A QUALITY MANAGEMENT SYSTEM?
A quality management system (QMS) can be expressed as the organizational structure, procedures, processes and resources needed to implement quality management. Our website provides templates for policies, procedures, processes, forms and system to enable you to build your QMS. The QMS required will depend on the type, nature and size of your organization.
Regulators and institutions (FDA, ISO, PIC/S, ICH, WHO) have taken steps to harmonize on a QMS Model with the following major sections:
1) Management Responsibilities
2) Resources
3) Manufacturing Operations
4) Evaluation Activities
We have translated the above categories graphically in our QMS road map (flowchart) below.
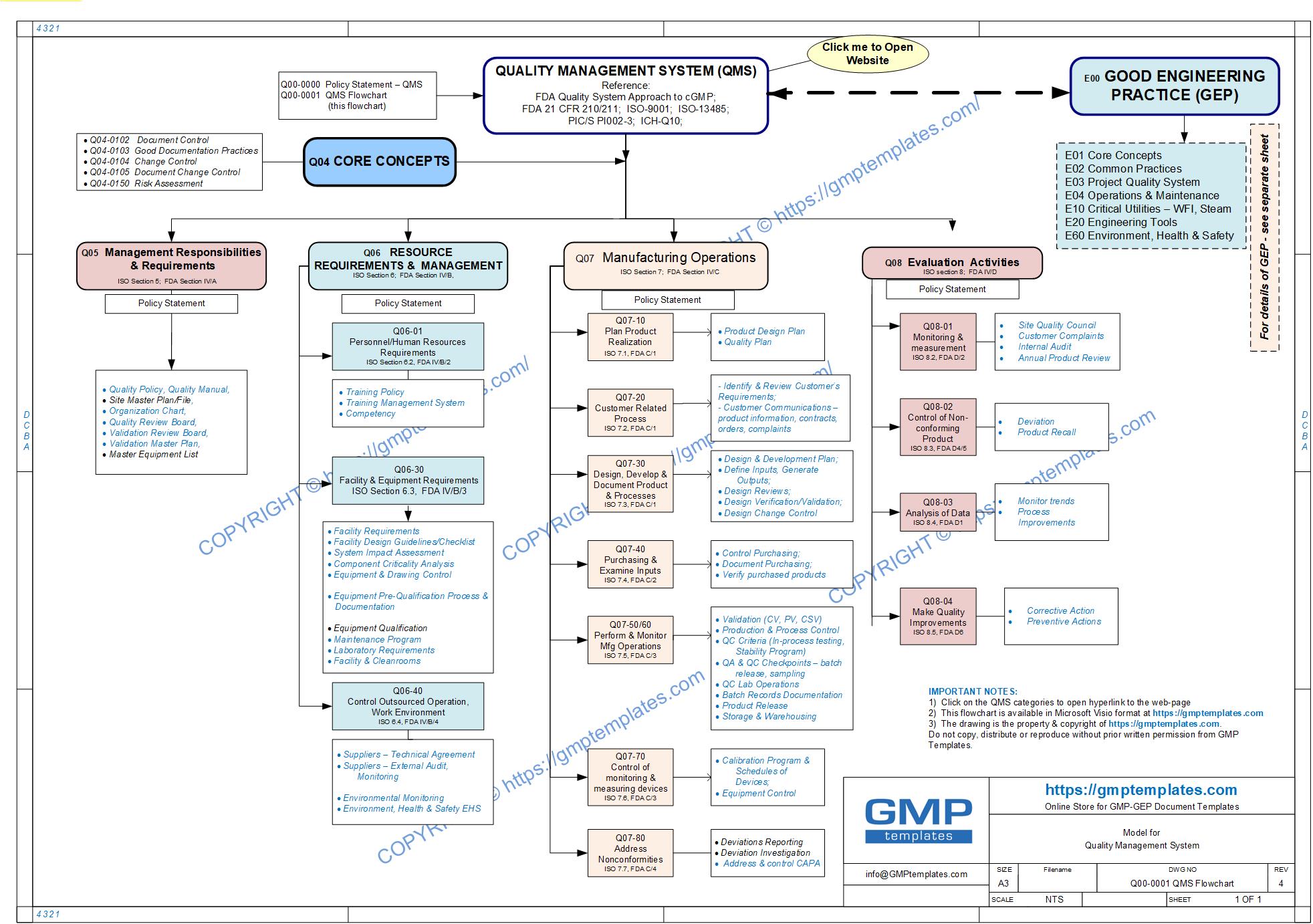
Q00-0001 Quality Management System Flowchart
Please open, download or print our QMS flowchart in PDF format for ease of reference – Q00-0001 Quality Management System – Flowchart-4
WHY DO WE NEED QMS?
A QMS enables an organization to achieve the goals and objectives set out in its strategy and policy.
A QMS:
– provides consistency and satisfaction in terms of a process, method, materials, equipment, etc,
– interacts with all activities of the organization, beginning with the identification of customer requirements and ending with their satisfaction, at every transaction interface.
A fully documented QMS will ensure the following requirements are met:
– The customers’ requirements – confidence in the ability of your organization to deliver the desired product and service consistently meeting their needs and expectations.
– The organization’s requirements – both internally and externally, and at an optimum cost with efficient use of the available resources – materials, human, technology and information.
Our QMS Flowchart is based on the merging framework of:
– FDA Guidance for Industry – Quality Systems Approach to Pharmaceutical CGMP Regulations.
– ISO 9001:standards for a Quality Management System (QMS)
– ICH-Q10 International Conference on Harmonization – Pharmaceutical Quality Systems
– PIC/s PE-009-16 Pharmaceutical Inspection Co-Operation Scheme – Guide to Good Manufacturing Practice for Medicinal Products
OUR WEBSITE OFFERS:
Our Team at www.gmptemplates.com with combined decades of experience, will help you to implement your QMS by offering industry proven procedures and standards. These are Standard Operating Procedures SOP for your Quality Assurance QA department.
YOUR NEXT STEP TO ESTABLISH QMS:
– State your Company’s philosophy and commitment to implement a quality management system. Then define the importance of QMS in the execution of manufacturing activities or projects to produce quality products. Please refer to Q04-00-01 Policy Statement on QMS.
– View and understand our QMS Flowchart and translate to your organization’s requirements
– Apply a “risk-based” approach – highlight and prioritize the procedures, methods and standards that you wish to introduce to your facility. Then select from our library of templates.
If you need help, please use the Enquiry Form in Contact Us.
The SUB-CATEGORIES within the Quality Management System are shown below.
CLICK TO OPEN EACH ONE.