E20-52 FACILITY DESIGN – GMP/GEP CHECKLIST – ARCHITECTURAL
$60.00
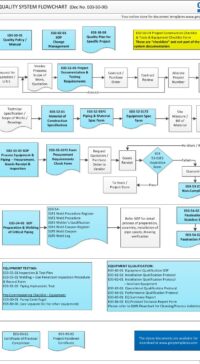
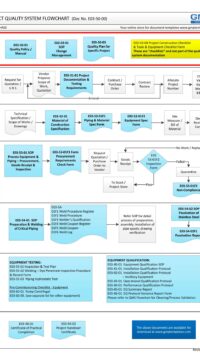
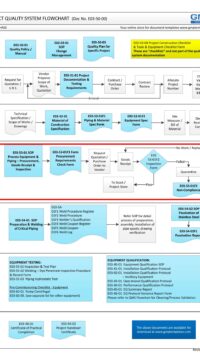
The architectural checklist consists of 120 design aspects in the life-cycle development, design, construction, testing, qualification and maintenance of facility components & building. Checklist will avoid costly retrofit and rework.
Purpose of Checklist:
The checklist consists of features & design criteria for a facility component or equipment or process that needs to be considered during the life-cycle phases of development, design, construction, testing, qualification and maintenance. This is to ensure the components in the facility are "fit for intended purpose", compliant to regulatory/statutory, easy and safe to operate and maintain. The result is to produce quality products.
The Architectural checklist:
- includes topics like layout, each of FDA CFR 211.42 Facility/Building requirements, building fabrics, people/material flow
- also include design criteria which is typical industry standard
- ensures that you have covered all expects in the design, construction, testing and maintenance of components in a facility.
- A missed item or design feature can lead to non-compliant, very expensive and cumbersome retrofit plus project delay.
- some checklists are built from statutory and regulatory requirements
The entire checklists in our library includes:
| No. of items in each separate Checklist | Number of Items in Checklist |
|---|---|
| Architectural | 120 |
| HVAC | 70 |
| Process Equipment | 155 |
| Process & Utilities | 150 |
| Electrical | 125 |
| Process Control/Automation | 285 |
| TOTAL | 905 |
The Checklists are supplied in MS Excel format for ease of entering data and sorting the items.