Description
What is this procedure about?
The purpose of this Standard is to define Good Documentation Practices as they pertain to all Good Manufacturing Practices (GMPs) records to ensure identity, authenticity and accuracy of records.

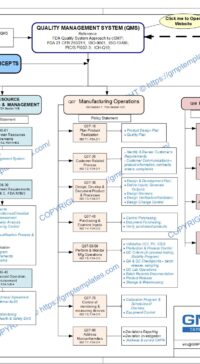
Q04-0000 Core Concepts applied to Quality Management System
Related Regulatory references:
In one form or the other, most regulatory authorities put a lot of emphasis on Good Documentation Practice as it is the core concept of a proper Quality Management System. Some of the regulatory requirements are:
- FDA Q7A Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients, Section VI. Documentation and Records
- When entries are made in records, these should be made indelibly in spaces provided for such entries, directly after performing the activities, and should identify the person making the entry.
- Corrections to entries should be dated and signed and leave the original entry still legible…..etc
- ICH Good Manufacturing Practice Guide for API Q7, Section 6 Documentation and Records
- PIC/S PE 009-8 (Part I) Guide to GMP for Medicinal Products Part 1, Chapter 4 Documentation:
- Good documentation constitutes an essential part of the quality assurance system.
- Clearly written documentation prevents errors from spoken communication and permits tracing of batch history.
- Specifications, Manufacturing Formulae and instructions, procedures, and records must be free from errors and available in writing.
- The legibility of documents is of paramount importance ….etc
What does procedure include?
- Definitions of terms and regulatory requirements
- 46 important points to prevent documentation errors – some are very basic human errors which can be easily avoided with “Right first time” principles
- A section is dedicated to Batch Records
Who needs this procedure?
- Quality assurance and Document Control for the management and control of documentation
- All departments that generate GMP documentation
Keywords:
Good Documentation Practice QMS Core Concept
Related Documents:
Q00-0001 Policy Statement – Quality Management System
Q04-0102 Document Control Procedure