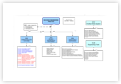
Description
Why do we need a Deviation Reporting Procedure?
One of the highest hidden costs and losses in productivity is managing deviations to normal process and quality. The negative effects when a deviation occurs are:
- further processing of product may stop,
- Supply-chain – product is held up,
- time and resource consuming investigations are required
- GMP non compliance raises risks to entire business.
For any Deviation from normal operations and quality, regulators as well as management expect the root cause to be eliminated or impact reduced:
- Identify the problem.
- Evaluate its magnitude, assess the risks to product quality and customer.
- Investigate and assign responsibility and timeframe.
- Analyze and document the root cause of the problem.
- Develop and implement CAPA
- Entire process is managed, monitored and controlled
The rewards to a well executed Deviation handling are improvement to your process and product, eliminate or reduce recurrence.
Associated Processes & Documents:
Q07-8001 Deviation Reporting Procedure (SOP) and Forms (5) (this document) guides you step by step through the process to handle Deviations. This document.
Q07-8002 Deviation Investigation Procedure guides you through the investigation process for significant Deviations.
Q07-8003 Laboratory Deviation Investigation Procedure guides you through the investigation process for significant Deviations in the Laboratory (including OOS).
There are two parts to this document:
- Part 1 – describes the rationale of why this procedure or standard is required, cross reference relevant regulatory clauses.
- Part 2 – the document template, appendices (3) and attached Forms (5).
- Includes examples of Events requiring a Notice of Event
- Classification of event/deviation
- A list of investigative Q&A to guide you through the Deviation investigation.
Related Regulatory references:
- FDA Guidance for Industry – Quality Systems Approach to Pharmaceutical cGMP Regulations; C4. Manufacturing – Address Nonconformities (full details in SOP)
- ISO-9001, Section 8.2.3 Monitoring and Measurement of Processes
- PICS PE 009-8 2009 01 Part1 Guide GMP Medicinal Products – Chapter 5.15 Production/Deviation and more (full details in SOP)
Keywords:
Deviation non-conformance failure discrepancy reporting investigation CAPA Corrective Preventive
Associated Documents:
Q07-8002 Deviation Investigation Procedure (by Deviation Investigation Team)
Q07-8003 Laboratory Deviation Investigation (including Out of Specification (OOS) Results)