Description
What is Management Responsibilities Policy in a Quality Management System?
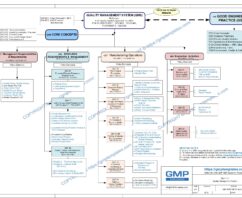
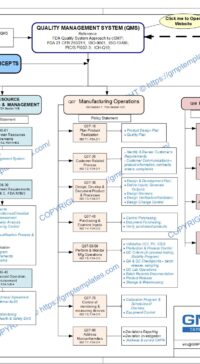
The four major components in a Quality System Model are:
- Management Responsibilities
- Resource Management
- Manufacturing Operations
- Evaluation Activities
Click here to open and download an Adobe PDF format q00-00-01_qms_flowchart
The purpose of the Management Responsibilities Policy is to provide an overview of our company’s policy to define Management’s role and responsibilities to support the quality system and produce quality products to satisfy Customers’ needs. The policies develop the path for subsequent procedures (SOP’s), systems and forms to complete the Quality System.

Management Responsibilities in Q05-0000 Quality Management System
Industry/Regulatory guidance and requirements:
- FDA Guidance for Industry – Quality Systems Approach to Pharmaceutical CGMP Regulations, September 2006, Section IV/A Management Responsibilities
- ISO 9001:2000, Section 5 Management Responsibility
- PIC/S Guide to GMP for Medicinal Products, PE009-8, Chapter 2
There are two parts to this document:
- Part 1 (2 pages) – Introduction to Template – describes the rationale of why this procedure or standard is required, cross reference relevant regulatory clauses.
- Part 2 (5 pages) – the document template and flowchart.
Associated documents:
Q00-01 Quality Management System
Q06-0001 Resources Requirements
Q07-0001 Manufacturing Operations
Q08-0001 Evaluation Activities