Description
PURPOSE
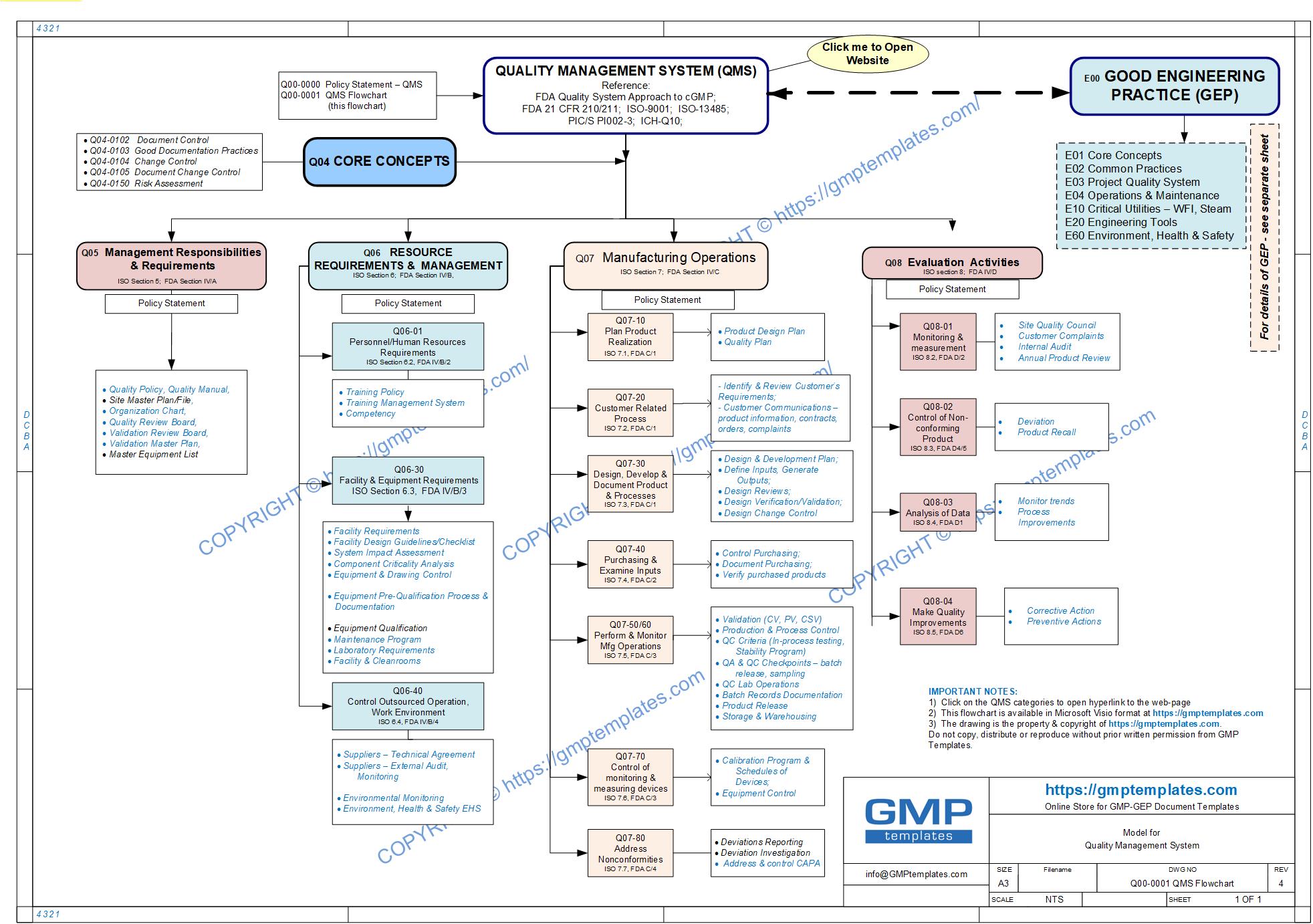
This whitepaper is intended as a guide to implementing a Quality Management System (QMS) based on:
- FDA Guidance for Industry ‐ Quality Systems Approach to Pharmaceutical CGMP Regulations (September 2006)
- ISO 9001 standards for a Quality Management System (QMS)
- ICH‐Q10 International Conference on Harmonization – Pharmaceutical Quality Systems
The whitepaper:
- explains the principles and the hierarchy of the system
- It only describes the typical processes as the QMS for each organization varies with its unique activities, processes and regulatory and customer requirements.
- provides the basic steps to implementation

Q00-0000 Quality Management System Category
Click here to View/Download/Print the QMS Flowchart in Adobe PDF format Q00-0001 Quality Management System Flowchart-3