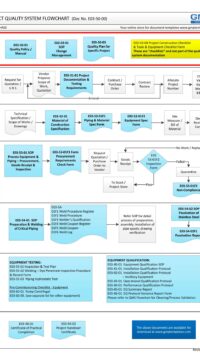
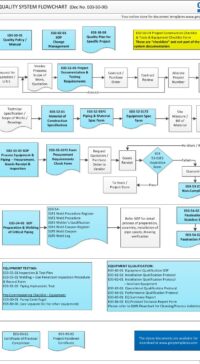
Description
This template together with the easy to follow flowchart provide the “big picture” road map and the rationale of a Quality Management System for the Regulated Industry (biotech, pharmaceutical, medical devices).
The purpose of this policy statement:
- is to state the company’s philosophy and commitment to implement a quality management system.
- is to define the importance of QMS in the execution of manufacturing activities or projects to produce quality products.
There are two parts to this document:
- Part 1 (2 pages) – describes the rationale of why this procedure or standard is required, cross reference relevant regulatory clauses.
- Part 2 (6 pages) – the document template and attached flowchart in Microsoft Visio format
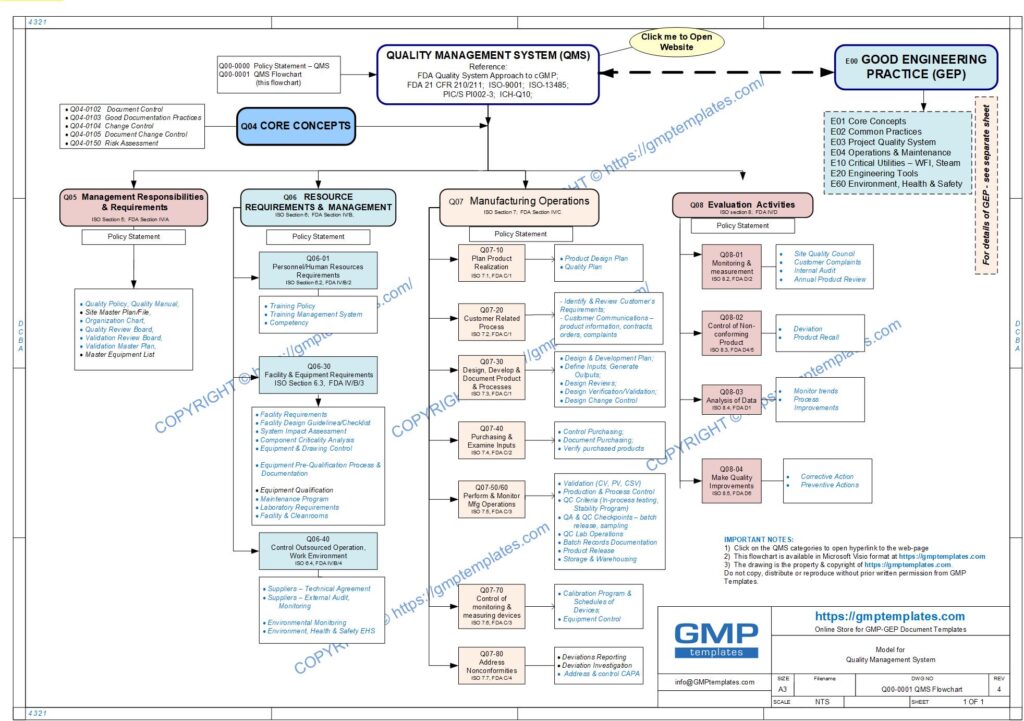
Q00-0001 Quality Management System Flowchart
Please Click Here to View/Download/Print a PDF format of the flowchart Q00-0001 Quality Management System – Flowchart-4
Related Regulatory References:
Industry groups have recently developed a consensus harmonization of quality systems and cGMP regulations.
This policy statement and QMS Flowchart is based on merging the framework of:
- FDA Guidance for Industry – Quality Systems Approach to Pharmaceutical CGMP Regulations (September 2006)
- ISO 9001 standards for a Quality Management System (QMS)
- ICH-Q10 International Conference on Harmonization – Pharmaceutical Quality Systems
Who needs this template?
Senior Management and Site Quality Head who need to design the overall QMS concept.
Related Documents:
Q00-0001 Quality Management System Flowchart