Description
Purpose of this procedure and form:
The purpose of this Standard Operating Procedure (SOP) is to define the requirements of the signature and initial register so as to ensure possible identification and traceability to personnel, who have acknowledged or authorized a particular task or process which could affect the safety, efficacy, identity, strength, purity or quality of the product manufactured. This SOP is part of the GMP Documentation Practice and Document Control.

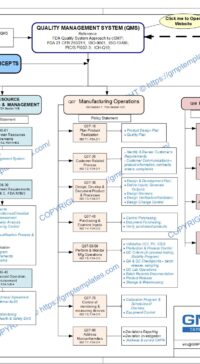
Q04-0000 Core Concepts applied to Quality Management System
Related Regulatory references:
- FDA Q7A Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients, Section VI. Documentation and Records
- ICH Good Manufacturing Practice Guide for API Q7, Section 6 Documentation and Records
- PIC/S PE 009-8 (Part I) Guide to GMP for Medicinal Products Part 1, Chapter 4 Documentation: Good documentation constitutes an essential part of the quality assurance system.
Who needs this procedure?
- Quality assurance and Document Control for the management and control of documentation
- All departments that generate GMP documentation
Keywords:
Documentation Document Control
Related Documents:
Q00-0001 Policy Statement – Quality Management System
Q04-0102 Document Control Procedure
Q04-0103 Good Documentation Practice