Description
This Standard Operating Procedure (SOP) covers changes to all aspects in a GMP business. Changes are essential to improve product quality and effectiveness, operation efficiency and business competitiveness.

Q04-0000 Core Concepts applied to Quality Management System
This “real-life” and well proven “19-Page + 7 Forms” SOP has been implemented in many established GMP facilities. Adjust the template to suit your activities and operations.
The supplied package has two parts:
- an introduction to the SOP, what are the governing Requlatory Requirements?
- The template + Appendices (6) + Forms (7)
PURPOSE:
- Change control systems is an integral part of the quality management system.
- To define the requirements and responsibilities of the change control and the process for the initiation, evaluation, review and approval of any changes that may have potential impact to product, process, regulatory fillings or validation status.
- To evaluate the effect of all approved changes on the quality of the products (i.e. purity, identity, efficacy and safety) prior to implementation.
- To ensure that all requested changes are carefully checked and completely documented and authorized.
SCOPE:
- This SOP covers changes to all GMP elements:
- Facility, Equipment, Utilities (e.g. critical utilities HVAC, Water Systems)
- Computer Hardware and Software (e.g. Quality System Database, GMP changes in MRP system)
- Support Systems (e.g., building management systems, preventative maintenance systems)
- Raw Materials & Products:
- QC Specifications & Methods
- Drug Products, API, Excipients, Starting Materials,
- Raw Materials Supplier / Source
- New or Discontinued Products
- Regulatory affairs – submission
- Manufacturing Process & Controls
- Manufacturing Process Steps; Validation
- Packaging:
- Artwork to packaging
- Primary Packaging Components
- Secondary Packaging Components that Impact the product Labelling and Packaging Equipment
- Warehouse & Storage Conditions, Stability Program and Expiration Dating
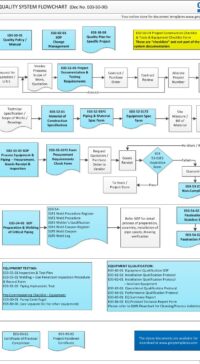
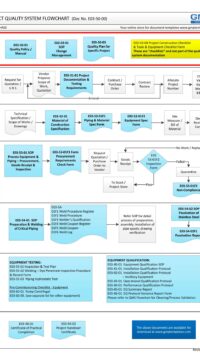
Change Control Process:
The SOP covers the full life cycle of a Change:
- Identification & Initiation of a Change
- Impact Assessment
- Regulatory Affairs and submissions
- Local site changes to Corporate Changes for multi-site operation
- Change Plan
- Categorizing a change
- Change authorization
- Tracking, Close-out
Appendices and Forms:
Process road map of Change Control, Change Categories, Change Control of Packaging / Manufacturing/ QC, Impact Assessment, Change Implementation/Verification, Tracking, Authorization, Addendum
Associated Process and Documents:
Q00-01 Policy Statement – Quality Management System
Q04-0101 Documentation Control
Q04-0111 Document Change Control Procedure
NOTE:
There are 3 Change Control templates in our website. The reasons for the three SOPs are due to their applications:
- E02-0201 CHANGE CONTROL (ENGINEERING) = most engineering changes occurs during the design stages prior to manufacturing and do not affect the “Critical Quality Attributes (CQA)” of the product. The SOP follows normal Good Engineering Practices (GEP) like revision control.
- Q04-0110 CHANGE CONTROL (this template) = follows all the steps expected from Regulatory Change Requirements – fully documented, executed and closed out
- Q04-0111 Document Change Control Procedure = this SOP relates to changes in GMP documents.