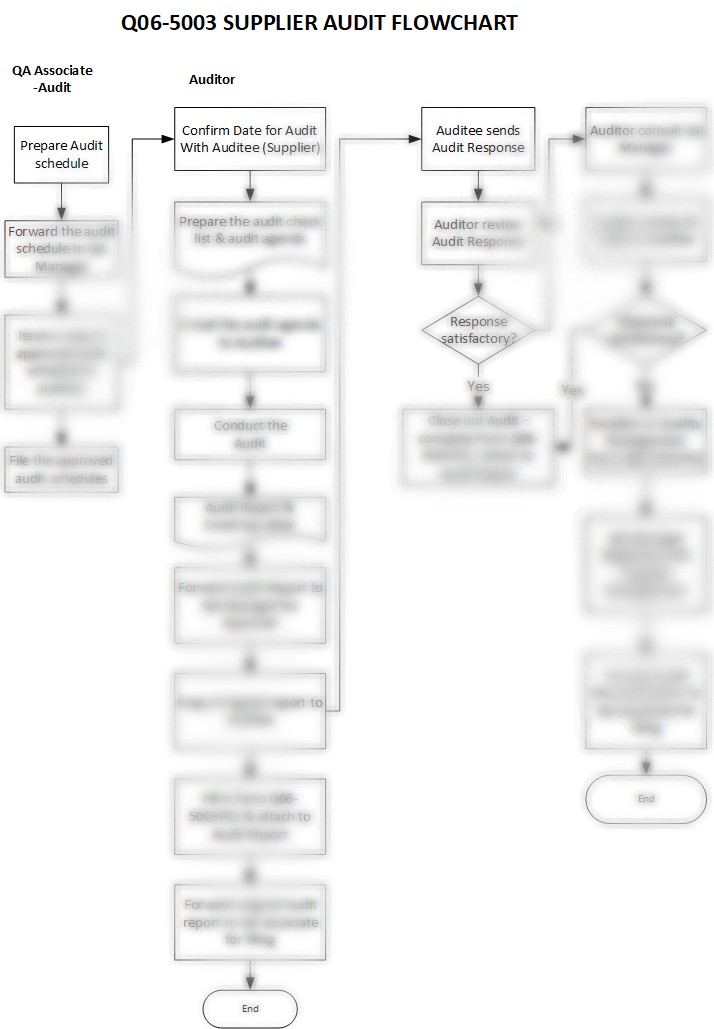
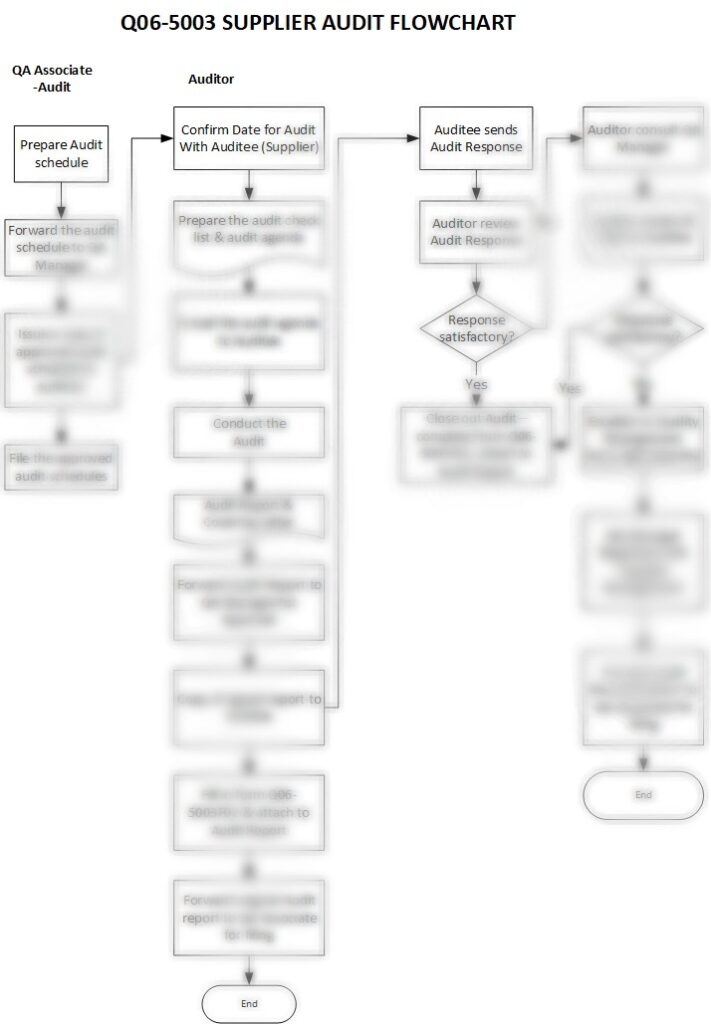
Description
Conducting a comprehensive audit of outsourced suppliers of Good Manufacturing Practice (GMP) materials and services is a crucial aspect of ensuring the quality, safety, and compliance of products in regulated industries such as pharmaceuticals, biotechnology, and medical devices. The purposes and anticipated results of such audits include:
Compliance Assurance:
Assurance that the materials and services provided meet the necessary quality and regulatory standards.
Risk Management:
Identify and assess potential risks associated with the outsourced suppliers, including risks related to product quality, safety, and regulatory compliance.
Quality Assurance:
Purpose: Evaluate the quality management systems, processes, and controls in place at the outsourced supplier’s facility.
Supply Chain Integrity:
Confidence in the reliability and security of the supply chain, reducing the likelihood of product recalls or safety issues.
Continuous Improvement:
Identify opportunities for improvement in both the outsourced supplier’s and the contracting company’s processes.
Implementation of corrective and preventive actions to enhance overall efficiency, quality, and compliance.
Relationship Building:
Improve communication, transparency, and cooperation, leading to better alignment of goals and mutual success.
Cost Efficiency:
Identification of opportunities for cost optimization without compromising quality or compliance.
Audit Trail Documentation:
A comprehensive record that can be reviewed by regulatory authorities and internal stakeholders, providing evidence of a commitment to quality and compliance.
Overall, the audit process aims to ensure that the outsourcing partners align with the contracting company’s quality and compliance standards, thereby contributing to the production of safe, effective, and high-quality products. Regular audits also contribute to the establishment of a robust quality management system throughout the supply chain.
To support the above purposes, we have developed the SOP procedures and form templates to perform, monitor and control your GMP Supplier Audit:

Q06-5003F01 Not Used
Q06-5003F02 Supplier Audit Summary Report
Q06-5003F03 Supplier Audit Request Form.
Q06-5003F04 Supplier Questionnaire Distributor Repack
Q06-5003F05 Supplier Questionnaire Distributor No-Repack
Q06-5003F06 GMP Questionnaire Manufacturer Excipients
Q06-5003F07 GMP Questionnaire Packaging Supplier
Q06-5003F08 Supplier Audit Schedule Template
Q06-5003F09 Supplier Pre-Audit Questionnaire