Description
Regulatory GMP requirements for Control of Outsourced Operations includes:
“The organization shall determine and apply criteria for the evaluation, selection, monitoring of performance, and re-evaluation of external providers, based on their ability to provide processes or products and services in accordance with requirements. The organization shall retain documented information of these activities and any necessary actions arising from the evaluations.”
Source: ISO9001 Clause 8.4 Control of externally provided processes, products and services.
“Any activity covered by the GMP Guide that is outsourced should be appropriately defined, agreed and controlled in order to avoid misunderstandings which could result in a product or operation of unsatisfactory quality…”
Source: PIC/s PE-009 Guide to GMP for Medicinal Products; Chapter 7 – Outsourced Activities
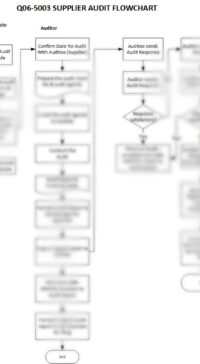
This Standard Operating Procedure (SOP) applies to all Suppliers of GMP goods and services.
Purpose – To describe the approval process for new Suppliers, and to describe the requirements for the ongoing management of these Suppliers.