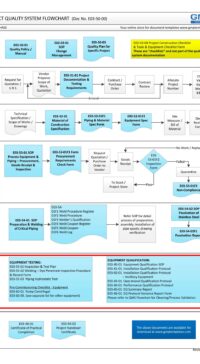
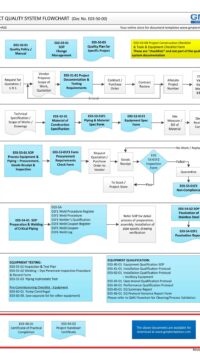
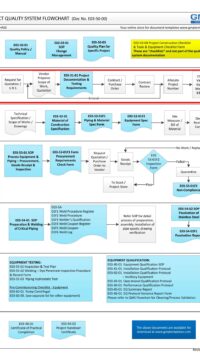
Description
Purpose of this guide:
- is to identify and define design features and typical industry practices
- to note design elements which must be incorporated within Pure Water System (PWS) and Water-for-Injection (WFI).
- provides a logical checklist for a WFI system. Any re-work or rectification of design and construction faults in these systems is a costly and time-consuming process, which usually has a severe impact on production and supply chain.
- it is essential to review each activity critically on an ongoing basis to insure that the best option and no errors are built into the system.
- The guideline can also used as a gap analysis for existing WFI system
Table of contents & Sample Pages:

E10-0102 WFI Design Guidelines-Table of Contents
Associated Industry/regulatory guidance and requirements:
- ISPE Baseline Guide – Water & Steam Systems
- FDA Inspection Guide High Purity Water (7/93)
- PIC/S Guide to GMP for Medicinal Products Part I
- US, BP and European Pharmcopeia
Keywords:
High purity purified water system Water-for-Injection WFI Design Guideline critical utilities
Who needs this document?
Vendors – equipment fabricators and manufacturers; mechanical piping contractors
Projects, Validation, Engineering department
Related Documents:
E10-01 Purified Water System – Design Input
E10-03 Purified Water System – Design Estimate (water usage pattern, sizing capacities of treatment plant, storage and piping)