Description
What is a GMP-Project Quality System?
A GMP production facility or clinical trial materials pilot plant is for the manufacture of pharmaceutical products. It includes the manufacturing space, the storage warehouse for raw and finished product, and support lab areas.
A GMP-Project is when the activities and deliverables are carried out in GMP environment and have the potential to affect the quality of the product manufactured in the facility. Executing a GMP-Project can be approached from the Client’s or Contractor’s perspective. For flexibility of both parties, this document can be adjusted to suit both the Client and Contractor. The end result remains the same, that is to ensure the project deliverables are met and ensure quality, compliance, cost and time benefits are maximised.
What is this process and document about?
In order for a project to meet the expected outcomes, a quality management process helps to implement quality assurance and control measures. With the added regulatory requirements for a GMP Project, a Project Quality System is a must to deliver quality, compliance, cost and time benefits to a project.
The purpose of this Policy Statement is to confirm our commitment to meeting the quality standards typically expected in the delivery of Projects. It is not the intent of this Project Quality System to take the place of an overall Quality Management System.
This document has 2 parts:
- Part 1 – describes the rational of why this procedure or standard is required, cross reference relevant regulatory clauses. (3 pages)
- Part 2 – the Policy Statement and attachment (3 pages)
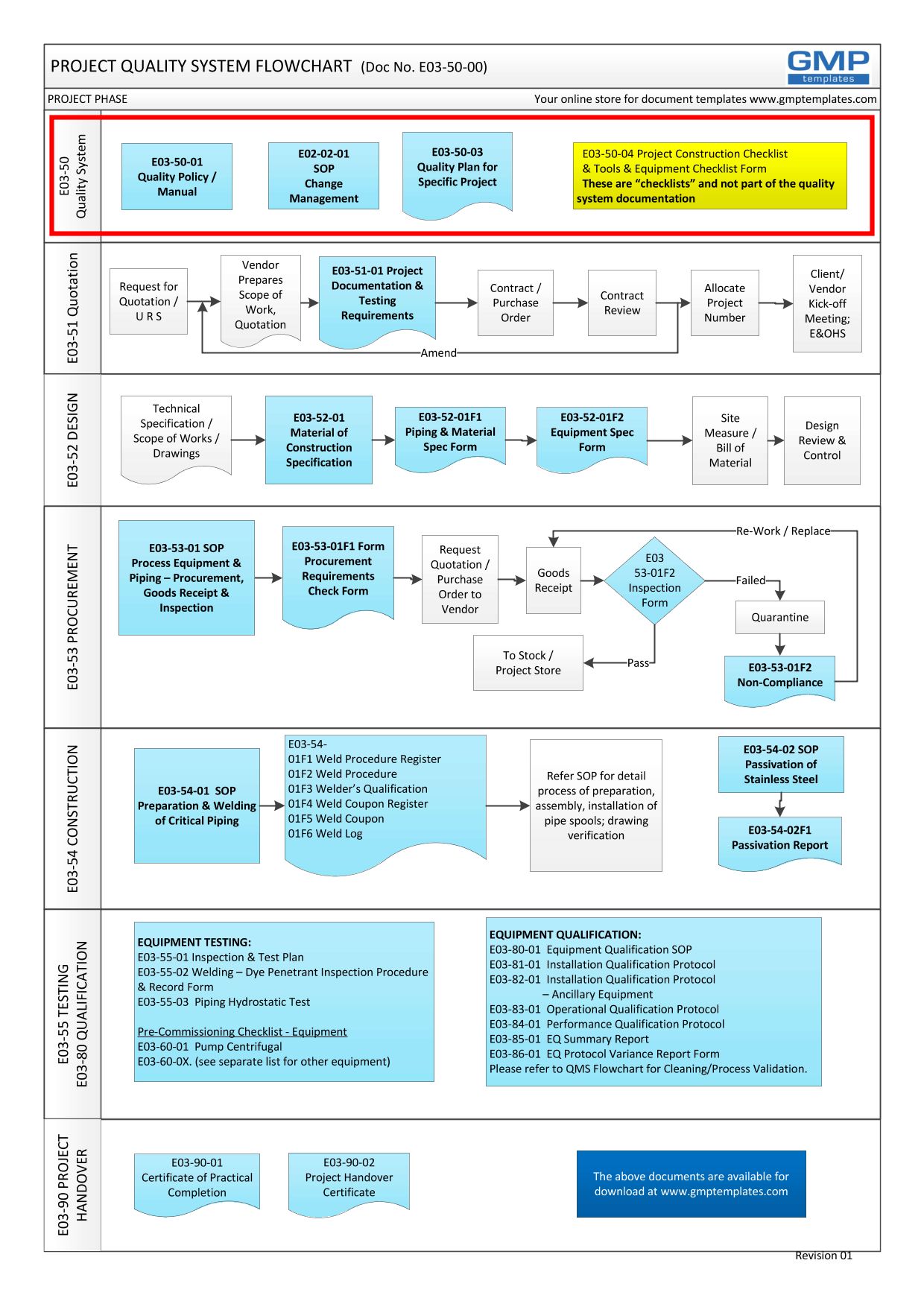
The Project Quality System that this Policy Statement relates to is shown below and covers the entire project GMP life-cycle:
- The core concepts of a quality system – eg Change Management and Project Quality Plan have to be established at the fore front. This will set the expectation from all parties.
- Project Contract Stage – from the gathering of Design Inputs, request for quotation, contract review, etc. This is the relationship building between the Client and Contractor.
- Design Stage – from conceptual design to detail design and review; development of material of construction, equipment and piping specification.
- Procurement – acceptance criteria for goods inspection, addressing non-conformance
- Construction – SOP for stainless steel welding, installation, passivation,
- Testing/Commissioning/Qualification – test plan and reports, IQ, OQ, PQ
- Project Handover process and reports
Click to open and download a FREE Project Quality System Model E03-50-00_Project_Quality_Sys_Flowchart
Please note that this document is the Policy Statement and does not include all the procedures and forms in the system.
Attachment included:
MS Visio model of a Project Quality System and Process (as shown in attached).
Associated Industry/regulatory guidance and requirements:
- ICH Q10 Pharmaceutical Quality System
- FDA Quality Systems Approach to Pharmaceutical CGMP Regulations
- PICS Guide GMP Medicinal Products
- ASTM E2500-7 Standard Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing System and Equipment
- ISPE Good Practice Guide: Good Engineering Practice (Core Concepts)
Keywords:
GMP Project Quality System Policy Statement
Who needs this document?
- Vendors – equipment fabricators and manufacturers
- Contractors – Design & construct turnkey; mechanical piping contractors
- Projects, Validation, Engineering department
- Quality Control
- Manufacturing
Related Documents or Topics:
E00-01-01 Good Engineering Practice (also refer flowchart E00-01-02)
E03-50-01 Project Quality System Policy – this document
E03-50-02 Project Change Control Form
E03-50-03 Project & Quality Plan (Concise)
E03-50-04 Project Construction Checklist
E03-51-01 Project Documentation & Testing Requirements
E03-52-01 Material of Construction Specification
- E03-52-01F1 Form: Piping & Equipment Material Specification Form
- E03-52-01F2 Form: Equipment Specification Form
E03-53-01 Procurement, Goods Receipt & Inspection
- Forms: Procurement Check; Inspection; Non-conformance Form
E03-54-01 Preparation & Welding of Critical Piping (6 Forms)
- Forms: 01F1 Weld Procedure Register; 01F2 Weld Procedure; 01F3 Welder’s Qualification; 01F4 Weld Coupon Register; 01F5 Weld Coupon; 01F6 Weld Log
E03-54-02 Passivation of Stainless Steel & Record Form
EQUIPMENT TESTING & QUALIFICATION:
- E03-55-01 Inspection & Test Plan & Forms
- E03-55-02 Welding – Dye Penetrant Inspection Procedure & Record Form (E03-55-02F1)
- E03-55-03 Piping Hydrostatic Test Procedure & Form
- E03-55-04 SOP EQUIPMENT QUALIFICATION (6 Forms):
- -04F1 Installation Qualification Protocol – Ancillary Equipment
- -04F2 Installation Qualification Protocol
- -04F3 Operational Qualification Protocol
- -04F4 Performance Qualification Protocol
- -04F5 EQ Summary Report
- -04F6 EQ Protocol Variance Report Form
E02-56-01 Project Handover – O&M Manual
E03-56-01F1 Practical Completion Form
E03-56-01F2 Project Handover Form