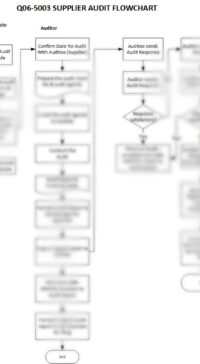
Description
Regulatory GMP requirements for Control of Outsourced Operations includes:
“The organization shall determine and apply criteria for the evaluation, selection, monitoring of performance, and re-evaluation of external providers, based on their ability to provide processes or products and services in accordance with requirements. The organization shall retain documented information of these activities and any necessary actions arising from the evaluations.”
Source: ISO9001 Clause 8.4 Control of externally provided processes, products and services.
“Any activity covered by the GMP Guide that is outsourced should be appropriately defined, agreed and controlled in order to avoid misunderstandings which could result in a product or operation of unsatisfactory quality…”
Source: PIC/s PE-009 Guide to GMP for Medicinal Products; Chapter 7 – Outsourced Activities
This Standard Operating Procedure (SOP) applies to all Suppliers of GMP goods and services.
Purpose:
- To avoid misunderstandings with contractors, that may result in product that is unsatisfactory, by ensuring contracts are defined, agreed and controlled.
- To outline the responsibilities and requirements between you and the approved third party Supplier.
ATTACHMENTS:
There are 3 x Forms included in this SOP:
(1) Q06-5002F01 Supplier GMP Agreement (5 pages)
(2) Q06-5002F02 Supplier Laboratory Services Agreement (6 pages)
(3) Q06-5002F03 Supplier Material Supply Agreement (9 pages)
These forms are comprehensive and well proven in the field. They are written and reviewed by various departments from Purchasing, Supply Chain, Regulatory Affairs, QA, QC, Engineering, Production.