Description
What is this process and document about?
- The biotech and pharmaceutical industry are looking for a whole range of process improvements to reduce the time and cost to build and validate facilities. One of the good practices that the industry, institutions and regulatory authorities are encouraging is Good Engineering Practice.
- Good Engineering Practice (GEP) is established methods and standards that can be applied throughout an engineering activity, project/equipment life-cycle to ensure delivery of appropriate and cost effective solutions.
- For Facility & Equipment, more emphasis is now placed on “quality at source” that is, driving “Quality” at grass roots engineering practices. A disciplined engineering system will provide a solid foundation to the whole process of producing quality products and meeting regulatory compliance.
- GEP is one of the core concepts to streamline the Validation process – as explained in ASTM-E2500.
This document has 2 parts
- Part 1 – describes the rationale of why this procedure or standard is required, cross reference relevant regulatory clauses
- Part 2 – the document template
Attachment –
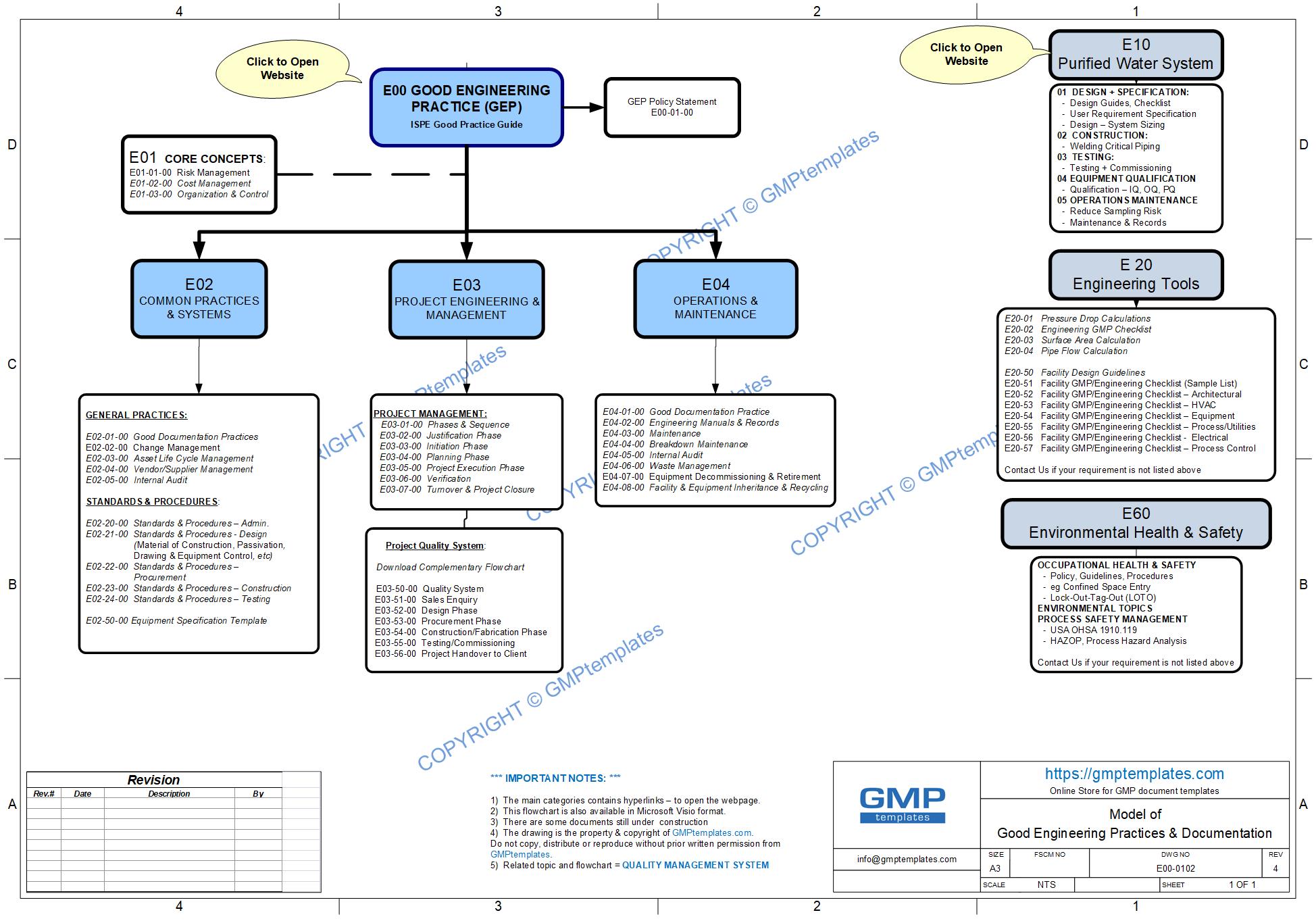
Good Engineering Practice (GEP) process map (flowchart) in MS Visio to guide you
Associated Industry/regulatory guidance and requirements:
- ISPE Good Practice Guide: Good Engineering Practice.
- ASTM E2500-7 Standard Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing System and Equipment.
Who needs this document?
Project Engineers and Managers, Maintenance, Validation Engineers, Vendors and Contractors.
Related Documents or Topics:
Q00-00-01 Quality Management System
E01-01-01 Risk Management (FEMCA)
E02 Common Practices & Systems
E03 Project Engineering & Management
E04 Operations & Maintenance