Description
What is this process and document about?
- This Standard Operating Procedure (SOP) provides guidance to the development, management and control of engineering drawings throughout its life-cycle.
- This document has 2 parts:
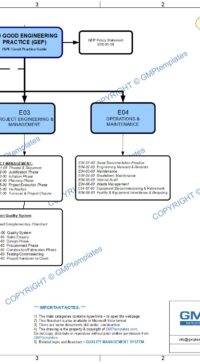
- Part 1 (4 pages) – describes the rationale of why this procedure or standard is required. The Good Engineering Practice flowchart shows the “big picture” of the role of Control of Drawings in good practice.
- Part 2 (15 pages) – the document template and two forms.
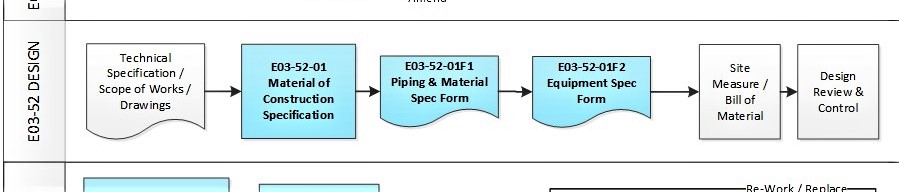
- The drawing control process begins with a methodical numbering and classifying (quality & safety impact) of the drawing, the application of Change Control, Maintenance requirements and finally the Life cycle of the drawing.
- This “grass roots” process is fundamental and the foundation to Good Engineering Practice, GMP, Validation and compliance to ensure the correct and “quality-impact” drawings and design is fit for “intended use” to produce quality products.
- Forms included:
- Drawing Classification Form takes you through a series of questions and answer to determine the impact of the drawing to GMP, Quality and Safety
- Drawing Register Template (MS Excel)
- Included Sections in Template:
- Purpose, Scope, Training, References and Associated Documents, Definitions/Acronyms, Responsibility, Procedures, Appendices and Attachments.
Industry/regulatory guidance and requirements:
- FDA (US) Q7A Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients, August 2001), Sections 5.1.
- PIC/S PE 009-8 2009 Guide GMP Medicinal Products – Part 1: 3.42, 4.5
- ASTM E2500-7 Standard Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing System and Equipment; Clause 6.6.3.3
- ISPE Good Practice Guide: Good Engineering Practice
Who will benefit from this document?
- Engineering department
- Quality Control
- Manufacturing
- Engineering Vendors and Contractors
Keywords:
Good Engineering Practice GEP Control of Engineering Drawings Drawing Register Classification GMP Drawing Equipment Control
RELATED TEMPLATES:
Other related Design processes and documents are in E03 Project Engineering
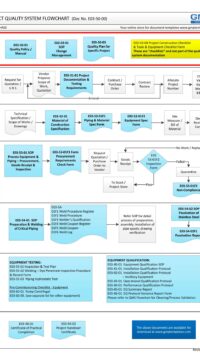
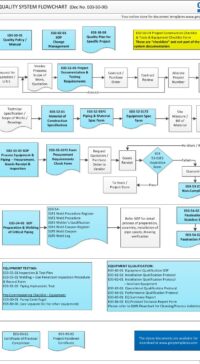
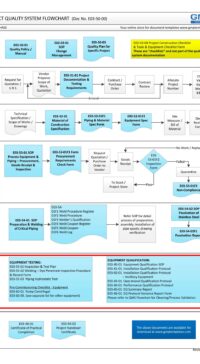
E03-50-00 Project Quality Sys Flowchart – Design