Description
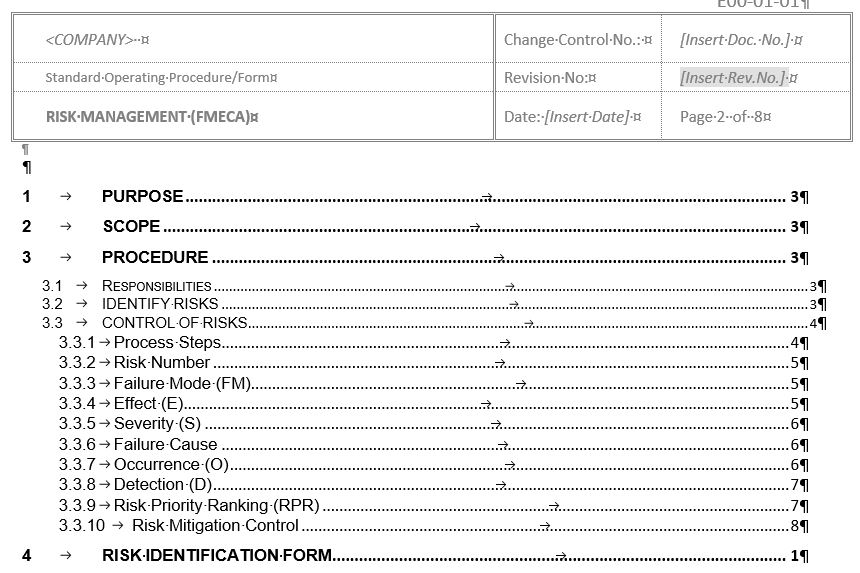
E01-0101 RISK MANAGEMENT (FMECA)
What is this process and document about?
- Risk management is a process to Identify, Assess, Prioritise, Mitigate, Preventive and Corrective Actions (CAPA) for the risk.
- Risk management is at the core of most Good Engineering Practice activity such that there is a balanced evaluation of risks against benefits. Where practicable efforts should be made to design out or minimize identified risks to an acceptable level (ISPE Good Practice Guide).
- This document addresses Risk Management using FMECA methodology.
- Failure Modes, Effects and Criticality Analysis (FMECA) methodology is used to rate and rank risks.
- This template includes 3 examples (Purified Water System, Cream Manufacturing and Project Risks (deliverables, quality, time and cost)) to illustrate the assessment process.
This document has 2 parts:
- Part 1 (3 pages) – describes the rational of why this procedure or standard is required
- Part 2 (12 pages) – the document template ·
Attachments & Forms included:
- Risk Identification Form – a risk identified by any personnel is recorded.
- Risk Control Form – this form guides you methodically through the risk assessment process.
Associated Industry/regulatory guidance and requirements:
- ASTM E2500-7 Standard Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing System and Equipment
- ISPE Good Practice Guide: Good Engineering Practice (Core Concepts)
- Reference only – ICH Q9 Quality Risk Management
Who needs this document?
- Any department who performs a process that involve a risk to product quality, safety, equipment, environment and the business.
Related Products:
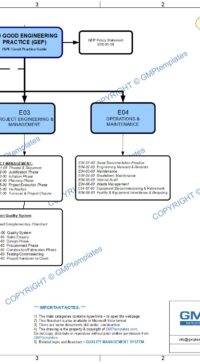
- E00-01-01 Policy Statement – Good Engineering Practice (also refer flowchart E00-01-02)
- E01-02-01 Cost Management
- E01-03-01 Organization and Control
- E02 Common Practices & Systems
- E03 Project Engineering & Management
- E04 Operations & Maintenance