A01 GMP Facility and Equipment Design Construction Testing Commissioning Decommissioning Qualification IQ OQ for Regulated Life Science Pharmaceutical industry inline with FDA ISPE PICs ISO9001
Showing 19–27 of 31 results
-
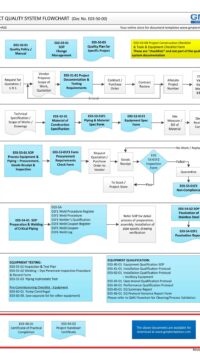
E03-9001 PROJECT PRACTICAL COMPLETION CERTIFICATE
$20.00 -

E03-9002 PROJECT HAND-OVER CERTIFICATE
$20.00 -

E10-0101 PURIFIED WATER SYSTEM – DESIGN INPUT INFORMATION
$55.00 -

E10-0102 DESIGN GUIDELINES FOR WATER-FOR-INJECTION WFI
$65.00 -

E10-0103 PURIFIED WATER SYSTEM – CAPACITY ESTIMATE
$90.00 -

E10-0401 SIMPLE TIP TO PROTECT PURIFIED WATER SAMPLING
$30.00 -

E20-51 FACILITY DESIGN – GMP/GEP CHECKLIST (SAMPLE LIST)
$0.00 -

E20-52 FACILITY DESIGN – GMP/GEP CHECKLIST – ARCHITECTURAL
$60.00 -

E20-53 FACILITY DESIGN – GMP/GEP CHECKLIST – CLEANROOM HVAC
$35.00



