Description
What is this process and document about?
- Control of Equipment is fundamental to Good Engineering Practice (GEP), Good Manufacturing Practice (GMP) and compliance to ensure the correct and well-maintained equipment is fit for “intended use” to produce quality products.
- This Standard Operating Procedure (SOP) provides guidance to the development, management and control of engineering and laboratory equipment and utilities throughout its life-cycle.
- A “Good Engineering Practice” (GEP) and Quality Management System (QMS) flowcharts provide the ‘big picture’ of the relations of this and associated process and documentation.
This document has 2 parts:
- Part 1 (5 pages) – this introduction describes the rationale of why this procedure or standard is required, cross reference to relevant regulatory clauses or industry best practices.
- Part 2– the standard operating procedure (SOP) template (12 pages) + 5 Forms
Attachments & Forms included:
There are 4 “must-have” form templates that will assist you to CONTROL & MANAGE your equipment and facility:
- Equipment Specification Datasheet – the critical and essential information regarding the equipment is kept in the datasheet. This is an interactive form guiding you through the life cycle of the equipment – from the initial performance requirements, design, construction, qualification through to maintenance and retirement.
- Master Equipment/Instrument List – this GMP document is a popular request from regulatory auditors. The two spreadsheets have columns for the essential information that an auditor will request.
- Instrument Calibration Control Form – similar to an equipment datasheet, this form relates to an instrument.
- Weighing Device Calibration Form – similar to an equipment datasheet, this form relates to a weighing device.
Associated Industry/regulatory guidance and requirements:
- FDA (US) Q7A Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients, August 2001), Sections 4.2, 5.1.
- PIC/S PE 009-8 2009 Guide GMP Medicinal Products – Part 1: 3.42, 3.44
- ASTM E2500-7 Standard Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing System and Equipment; Clause 6.6.3.3
- ISPE Good Practice Guide: Good Engineering Practice
Included Sections:
DOCUMENT TEMPLATE – Purpose, Scope, Training, , References and Associated Documents, Definitions/Acronyms, Responsibility, Procedures, Attachments.
Who needs this document:
- Any department who uses equipment, instruments & utilities in R&D, manufacturing, testing, laboratories, logistics.
- Engineering department
- Quality Control
- Manufacturing
- Engineering & Instruments Vendors and Contractors
Related Documents:
E00-01-01 Policy Statement on Good Engineering Practice
E02-02-01 Change Control (Engineering)
E02-21-10 Control of Engineering Drawings
E02-21-02 Drawing sheet Template – P&ID Symbols Sheet 1 of 2
E02-21-03 Drawing sheet Template – P&ID Symbols Sheet 2 of 2
E02-21-04 Drawing sheet Template – Isometric A3 Size
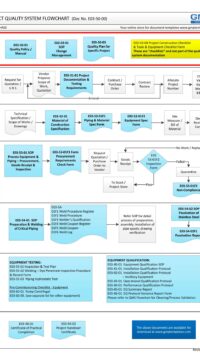
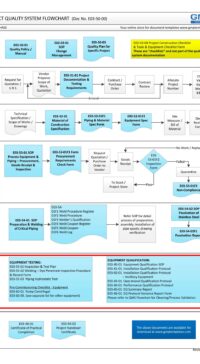
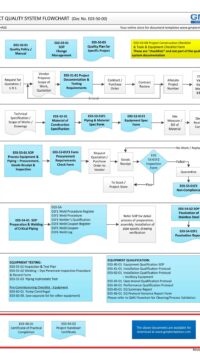
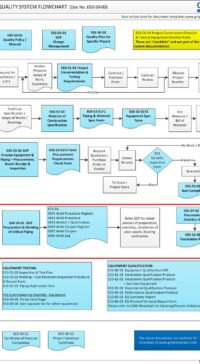
In addition, some of the typical DESIGN processes and documents for a small GMP design and construct project is shown below:
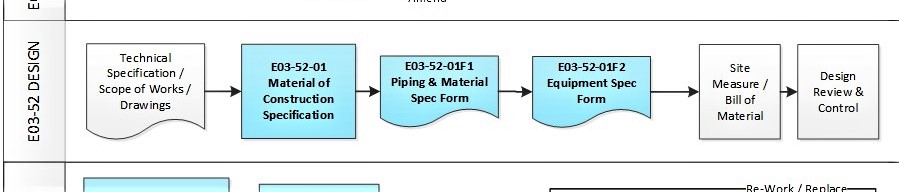
E03-50-00 Project Quality Sys Flowchart – Design
To view the entire E03-5000 Project Quality System Flowchart
Keywords:
Process equipment control, instrument, calibration GEP tagging identification equipment specification performance datasheet Master Equipment List Weighing device