Description
Note:
- Below is a white paper on a Simple Quality System for GMP Projects. It provides a simple process map and descriptions of the processes that are typically required for a medium size EPCMV mechanical project. It is also suitable for GMP Equipment and System manufacturers who design and supply to the pharmaceutical industry.
- A complementary PDF version is also available – click Add to Cart above
What is a GMP Project?
A project where the activities and deliverables are carried out in a GMP (Good Manufacturing Practice) environment and have the potential to affect the quality of the products manufactured in the facility. Good Engineering Practice is one of the core concepts to deliver a successful GMP Project.
What is a Project Quality System?
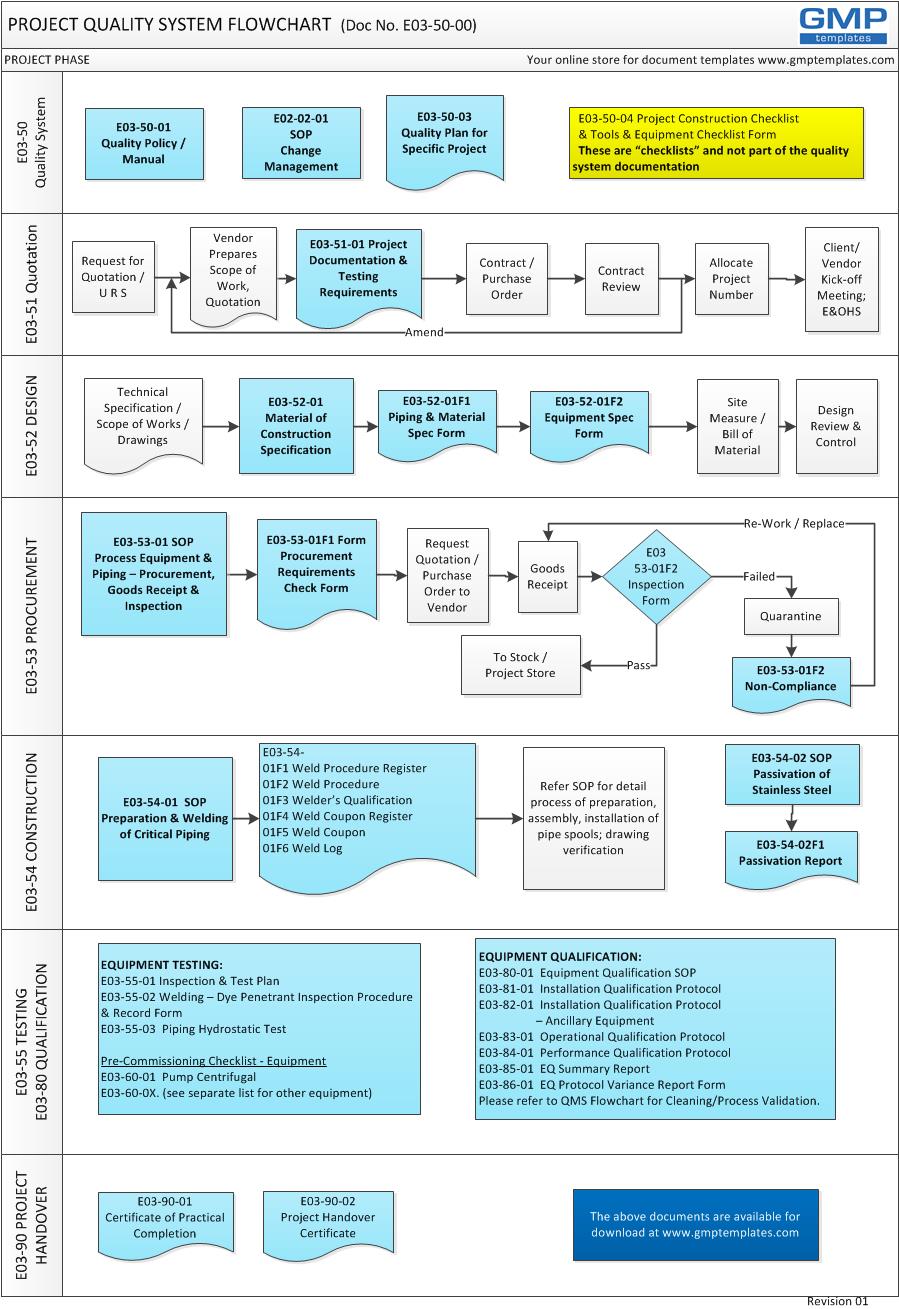
It is a system that describes the overall framework and processes, and the various elements (procedures, forms and resources) involved in improvements to projects. Improvements include quality of work, compliance, cost and schedule. The flowchart below illustrates typical phases in the definition and execution of a medium size EPCMV (engineering, procurement, construction management, validation) project.
Why do you need a Project Quality System?
Common weaknesses in executing GMP-Projects are:
- that the Regulatory/GEP/GMP requirements expected by the Client are not well defined and relayed to the Contractor
- Client as well as Contractor does not have a quality system in place – there is no baseline to benchmark
- the processes and expectations are not mapped out for all parties.
The quality system model will take you through each logical step to ensure deliverables to GMP Projects are met with maximum quality, compliance, cost and timeline benefits:
- set project quality standards, targets, deliverables and acceptance criteria ie Quality Policy and Plan
- define the Testing and Qualification processes to report how the required acceptance criteria are met
- it will minimize rejects, rework and project scope variations
- the processes and documentation generated are transportable from design through to equipment qualification – ie avoid repetitive documentation
SEVEN PHASES OF PROJECT EXECUTION & QUALITY SYSTEM:
1 QUALITY SYSTEM DEFINITION
This phase defines the ground rules of the project for the Client and Contractor/Vendor:
- Quality Policy statement – the purpose of a policy is to confirm both parties’ commitment to meeting the quality standards expected in the delivery of the project.
- Change Management – This procedure describes the system to control and manage all changes that can impact on the scope, deliverables, schedule and cost of a project. In particular if the change has a direct impact on the quality of the product manufactured in the facility. It ensures these changes are clearly identified, documented, reviewed and approved
- Quality/Project Plan – is a set of activities planned at the beginning of a project that helps achieve Quality in the Project being executed.
2 REQUEST FOR QUOTATION
The scope of the project and User Requirement Specification have to be specific and well documented to avoid disagreement at a later stage. The Project Documentation & Testing Requirements procedure and forms covers the following aspects:
- the scope of responsibilities of Client and Contractor.
- the critical documents and design parameters relevant to the project
- the activities and documentation to be produced by both parties
- the acceptance criteria and documentation for testing, commissioning and qualification
- in addition, the economics, business criteria and quality factors are built into this Project Documentation & Testing Requirements. This important process ensures cost vs quality vs timeline are well balanced and requires the contributions from all parties – QA, QC, Validation, Manufacturing and the Contractor.
3 DESIGN & SPECIFICATION
Depending on the size and complexity of a project, the Design Phase may include an elaborate Technical Specification, Synonymous with the saying “Garbage-In equates Garbage-Out”, the information from the Project Documentation & Testing Requirements (above) is the input to the design process and therefore has to be well defined:
- Material of Construction Specification is fully defined and concurred
- Equipment Specifications for major items are specified for procurement
- Piping Specification for process, critical and non-critical utilities are defined
- Electrics and Controls Specification for specific equipment and overall project are defined.
In addition, the purpose of these documents is to ensure technical specifications and acceptance criteria for a project are relayed to materials/equipment suppliers and sub-contractors.
4 PROCUREMENT
All the requirements and specifications developed above are used for procurement and as acceptance criteria for the equipment or facility:
- A Project Procurement & Inspection Procedure (SOP) and the associated forms provide a standard procedure to procure, inspect and control incoming materials or equipment ready for fabrication and installation
- A Project Procurement Form is used as a tool to convey the Project Documentation & Testing Requirements to suppliers. “Controlled” material and equipment require extra attention to details, identification and handling.
- A Project Inspection Form is use to verify conformance of the incoming materials as specified and the handling of non-conformance material.
5 CONSTRUCTION
The Construction process can vary a great deal on the scope and complexity of a project. For simplicity, the flow chart only covers the fabrication of mechanical items and piping:
- A Preparation and Welding of Critical Piping Procedure (SOP) and associated forms (refer flow chart above) describes the process of handling, identification, preparation, welding and documentation of piping and components. Attention to detail is of prime importance to prevent mix-ups, cross contamination and ease of Qualification.
- A Passivation of Stainless Steel process ensures the passive layer is re-instated to minimize corrosion.
6 TESTING & QUALIFICATION
This process is one of the biggest gaps in GMP-Project interpretation between Client and Contractor. Resources requirements, Quality, timeline and cost blow-outs are evident in many GMP-Projects. Regulatory, institutions, Vendors and Pharmaceutical Manufacturers are working together to minimize them.
- A well developed Test Plan and procedures (refer flowchart) ensure acceptance criteria is tested and met.
- Equipment Pre-commissioning Checklist – due diligence is practiced here to ensure safety to operators and “fit-for-intended purpose” prior to start-up.
- Equipment Qualification is mandatory for GMP equipment and systems as required by regulatory. The components for Qualification are shown in the flowchart above.
The above system and document templates are available for download on this webiste
Note: A PDF version is available – click Add to Cart above